Back to Journals » Infection and Drug Resistance » Volume 16
A Rare Case of Neuralgic Amyotrophy Associated with Brucella Infection
Authors Zhang G, Yan F, He F, Liu D, Wang L
Received 4 December 2022
Accepted for publication 27 January 2023
Published 23 February 2023 Volume 2023:16 Pages 1145—1151
DOI https://doi.org/10.2147/IDR.S400228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Gaozan Zhang,1 Fenfen Yan,1 Fei He,1 Dingxi Liu,2 Libo Wang1
1Department of Neurology, China-Japan Union Hospital of Jilin University, Changchun, 130031, People’s Republic of China; 2Department of Clinical Medicine, Zunyi Medical University, Zhuhai, 519041, People’s Republic of China
Correspondence: Dingxi Liu; Libo Wang, Email [email protected]; [email protected]
Abstract: Multiple micro-organisms associated with Neuralgic Amyotrophy (NA) have been reported and Brucella species should be an important and overlooked infectious cause or trigger.We report a rare case of neuralgic amyotrophy associated with Brucella infection and is believed to be the first such case report in China. A 42-year-old male with brucellosis was confirmed serologically, who presented recurrent fever and fatigue and suddenly developed severe pain in the right shoulder within one week, followed by the inability to lift and abduct the proximal end of the right upper limb. Based on typical clinical manifestions, MRI neuroimaging of the brachial plexus and neuro-electrophysiological studies to confirm a diagnosis of NA and presented spontaneous recovery during this period, immunomodulatory treatment with corticosteroid or intravenous immunoglobulin had not been attempted, leaving a serious movement disorder in the right upper limb. Even rare, NA and other neurobrucellosis forms should be considered as complications of Brucella infection.
Keywords: Brucella infection, neuralgic amyotrophy, immune-mediated nature, diagnosis and treatment
Introduction
Neuralgic amyotrophy (NA), also described as Parsonage Turner syndrome or idiopathic brachial plexopathy, is an auto-immune multifocal peripheral nervous system disorder,1 that usually affects the upper limbs. The classic presentation is a patient with acute onset of unbearable pain with rapid multifocal weakness and atrophy unilaterally in the shoulder and arm muscles, which mainly involves the upper and middle trunk of the brachial plexus.2 Any immune-related factor can trigger NA, including infection, vaccination, immunotherapy, recovery from surgery, pregnancy or childbirth, trauma or psychological distress.1,3 Infections are believed to trigger an immune-mediated reaction leading to neuralgic amyotrophy in 43% of cases3 and reported micro-organisms associated with NA keep increasing. Various viral, bacterial and fungal infections, including herpes simplex virus, Epstein–Barr virus, cytomegalovirus, SARS-CoV-2, Mycoplasma pneumoniae, Escherichia coli, Borrelia burgdorferi, Bartonella henselae and Aspergillus species etc, can trigger neuralgic amyotrophy.4,5 Neuralgic amyotrophy triggered by Brucella infection is extremely rare and to our knowledge, there has been one previous report on neurological syndromes of brucellosis in 19886 and another report on neuralgic amyotrophy of the shoulder associated with systemic brucellosis in 1992.7 Here, we present a patient with brucellosis who developed severe pain in the right shoulder within one week, followed by the inability to lift and abduct the proximal end of the right upper limb. Initial evaluation tracking for brucellosis lead to peripheral musculoskeletal involvement is incorrect, subsequent investigations for guidance to confirm neuralgic amyotrophy associated with Brucella infection.
Case Presentation
A 42-year-old male with recurrent fever and fatigue on 2th December 2021 thought he had a common cold. However, he suddenly developed severe pain and weakness in the right upper limb within one week and was subsequently unable to lift up or abduct the proximal end of his arm. The symptoms were continuous, while essential treatment was not being carried out. The patient was admitted to the hospital with recurrent fever, sweating, and fatigue on 6th January 2022 in the local area, and reported that because of persistent pain and weakness in his right upper limb and was unable to lift up or abduct the proximal end of right shoulder. Because of consumption of incompletely sterilized dairy products, depended on Rose Bengal Plate Test (RBPT)-positive and second specific test validated by a titer of Serum Agglutination Test (SAT) was 1:200 (SAT titers of ≥1:160 are considered diagnostic when coupled with a compatible clinical presentation),8 he was diagnosed with brucellosis at the local Centers for Disease Control and Prevention and was given anti-brucellosis therapy (monotherapy with Metacycline, 300mg/time, 2 times/d). Based on clinical history, clinician concluded that the patient had developed shoulder arthritis and possible triggers related to Brucella infection and longer courses of antibacterial treatment with analgesic treatment are recommended. One month after, this patient’s symptoms were progressively aggravated with the right upper limb mainly manifesting as right shoulder weakness during shrugging, right upper limb lifting of 10° and abduction of 5°, and slight numbness of the right palm, accompanied by persistent right shoulder pain, Thus he came to the neurology department of our clinic.
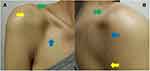
On admission, a detailed physical examination was performed (Figure 1). The patient had a temperature of 36.2 °C, clear consciousness, no abnormalities on cranial nerve examination, a right square shoulder deformity, mild atrophy of the Supraspinatus, infraspinatus, teres minor, deltoid, pectoralis major and latissimus dorsi muscles, the strength of his proximal right upper limb was 1/5, the strength of his distal right upper limb was normal, sensory assessment showed reduced sensation over the right palm, and biceps reflex, triceps reflex, radial membrane reflex were absent in affected right upper limbs. The positive laboratory findings included: peripheral blood examinations: WBC 2.06× 109/L, PLT 48× 109/L, liver function examinations: AST 62.12 IU/L, GGT 201.92 IU/L, LAP 86.46 U/L, GLDH 19.68 U/L, ALP 196.68 IU/L. Leukopenia, low platelet count and elevated liver enzymes all suggested that the patient was in the acute or subacute phase of a Brucella infection.9 The patient was treated positively treated for brucellosis according to the original antibiotic regimen.
On the third day of admission, only physiotherapy and symptomatic supportive treatment, such as ibuprofen was given, the patient reported that the pain in his right shoulder had decreased, his right upper limb could be lifted upwards by 30–40° and he had approximately 30° of abduction. To clarify the cause of severe pain and weakness of the shoulder and arm muscles, We performed MRI neuroimaging of the bilateral brachial plexus nerves using high-resolution T2-weighted, fat-suppressed sequences. The examination revealed irregular hyperintense signal abnormalities in the multifocal distribution regions of the brachial plexus (Figure 2) and signs of muscle denervation (diffuse hyperintense signal abnormalities) were found in the right supraspinatus, right infraspinatus, right teres minor, right deltoid, right pectoralis major, right latissimus dorsi, right biceps brachii, and right triceps brachii muscles (Figure 3). Electrophysiological studies (Figure 4) were further carried out to clarify the extent of the patient’s peripheral nerve injury, and the motor conduction velocity (MCV) examination showed that the right median nerve action potential (MNAP) was decreased. Compound muscle action potential (CMAP) examination showed that the latency (Lat) of the right musculocutaneous nerve, right axillary nerve, right radial nerve, right suprascapular nerve, right lateral thoracic nerve and right thoracodorsal nerve were in the normal range, and the amplitudes (Amp) were decreased. EMG displayed “+” spontaneous electric activities of the right abductor pollicis brevis, right deltoid and right infraspinatus muscles during the resting state and spontaneous generating activities of the right interosseous, right extensor digitorum communis, right biceps brachii, right pectoralis major, right infraspinatus, right triceps brachii and right latissimus dorsi muscles during the resting state, with the maximum forced recruitment of a small amount of MU, simple patterns and mixed simple patterns. The above positive results indicated neurogenic changes in the right upper limb, along with neuroaxonal damage. Based on medical history, MRI neuroimaging of bilateral brachial plexus and Neuro-electrophysiological studies, We properly speculated Brucella infection are believed to trigger an immune-mediated reaction leading to Neuralgic amyotrophy.
Due to the possible immune-mediated nature, Given the circumstantial evidence of a beneficial effect of immunomodulatory treatment with corticosteroid or intravenous immunoglobulin on Neuralgic amyotrophy. High-dose corticosteroid treatment may reduce the average duration of initial pain and improve functional recovery in the acute phase of neuralgic amyotrophy.10 However there was a long delay from symptom onset to treatment of approximately 3 months, corticosteroid treatment was not given. Because the patient presented spontaneous recovery during this period and difficulty in obtaining reimbursement of IVIG treatment cost for off-label use, intravenous immunoglobulin had not been attempted. On the sixth day, the patient was discharged from the hospital and was given anti-brucellosis therapy for 3 months with successive follow-up in serological tests is negative. The mobility of the right upper limb was a moderate degree of spontaneous recovery, and the patient had 110° of upwards lift and 80° of abduction. Although there was no recurrence of painful weakness in the right shoulder or numbness in the right palm, leaving a serious movement disorder in the right upper limb that have a negative effect on the patient’s nutrition, or the ability of the patient to obtain clothing, shelter or transportation.
Discussion
Brucellosis are responsible for one of the most widespread bacterial zoonoses caused by gram-negative intracellular bacterium Brucella spp and its main routes of infection include direct or indirect contact with mammalian infectious sources such as cattle, sheep, pigs and dogs, consumption of unpasteurized dairy products and raw meat, and infections of unknown causes,11 which has a wide spectrum of clinical manifestations, the symptoms often lack specificity and can mimic other infectious and non-infectious diseases. Acute or long-term chronic infections can cause multisystem involvement and involvement of the peripheral nervous system is rare, manifesting as cranial nerve injury, carpal tunnel syndrome, radiculitis, Guillain-Barre Syndrome (GBS) and NA etc. A extraordinarily rare presentation associated with Brucella Infection may cause a delay in diagnosis presenting a great challenge to clinicians, such as NA.
The diagnosis of neuralgic amyotrophy is mainly based on typical clinical manifestations.12 Initially, 96% of the patients have neuropathic pain, mainly involving the unilateral shoulder girdle muscle, with the duration varying from one day to two months. About 10% of the patients suffer from severe persistent pain;2,3 Subsequently, muscle weakness and atrophy accompanied by loss of axon reaction are usually observed within 2–4 weeks after symptom onset;13,14 Finally, within 3 months of the disease progression, the patients without immunotherapy may show unidirectional spontaneous recovery.15,16 Neuro-electrophysiological examination can provide a characteristic basis and a fixed neurogenic pattern can be observed when the disease progresses to 2–4 weeks12,16 :single unit pattern to only simplified interference pattern with high firing rate of motor units (multifocal axonal loss from peripheral origin) and numerous denervation signs (positive fibrillations and sharp waves).MRI neuroimaging of the brachial plexus can also observe that the T2 high-signal area of the affected brachial plexus branches and their innervated muscles is related to axonal loss.17 The pathological results obtained from surgical cases suggest that the affected brachial plexus branches have inflammatory cell infiltration complicated with severe axonal loss.18
We report a patient developed unilateral NA with possible cause or trigger related to Brucella infection. The patient presented typical infection with Brucella and suddenly developed severe pain in the right shoulder within one week, followed by the inability to lift and abduct the proximal end of the right upper limb, which was initially misdiagnosed as peripheral arthritis, and continued to be treated with antibiotics, but the condition became increasingly severe without relief. Based on immune-triggering event preceding NA, clinical history, MRI neuroimaging of the brachial plexus and neuro-electrophysiological detection, neuralgic amyotrophy can be regarded as a rare complication of brucellosis, thus needs to be considered as an important differential diagnosis in endemic areas.
The association between NA and Brucella infection is likely related to an autoimmune process and is largely unknown and understudied. A possible mechanism for Brucella infection triggering NA is the molecular mimicry theory, comparable with Guillain–Barré syndrome, which is defined as the dual recognition of a microbe’s structure and an antigen of the host by a single B- or T-cell receptor, and this is the mechanism by which infections can trigger cross-reactive antibodies or T cells that can lead to autoimmune diseases.19 At present, the lipopolysaccharide of Brucella has been proven to have a ganglioside-like structure. Anti-GM1 ganglioside antibody was detected in the serum of mice immunized with Brucella, and this may occur by molecular simulation with peripheral gangliosides, leading to demyelination and axonal degeneration.20 Alternatively, NA may be triggered by the (re) activation of plexus-reactive T-cell clones. The research of Suarez et al on inflammatory-immune brachial plexus neuropathy has revealed that inflammation-induced CD8+T lymphocyte infiltration may participate in the activation of T cells in the brachial plexus.21 Mostly reported micro-organisms as a trigger for neuralgic amyotrophy including Brucella are intracellular organisms and are known to trigger a CD8+ T-cell-mediated immune response,4,22 suggesting a possible mechanism for T-cell-mediated autoimmunity. In our case or previous case reports on neuralgic amyotrophy triggered by intracellular microorganisms, antibiotic therapy is often ineffective or has a good clinical response to immunotherapy is consistent with an immune-mediated reaction to infection preceding NA, which may provide evidence for infection-mediated immune response.
To date, treatment plans for NA include analgesia, immunomodulation, physiotherapy, and surgery. For acute‐phase treatment, analgesics, including non‐steroid anti‐inflammatory drugs, opioid medications, and antipsychotics, are essential for active pain control.15 In light of potential immune mediation and presumably valid immunotherapy evaluation, immunomodulators such as corticosteroids and intravenous immunoglobulins are recommended for improved prognosis.23 On the other hand, physiotherapy, predominantly motor skill exercises, plays a vital role in the chronic‐phase treatment and rehabilitation of NA. Neuroradiological research on this topic has reported potential morphological changes of the involved nerves, early inflammation‐induced nerve expansion, and focal hourglass‐like nerve constriction due to nerve adhesion and fixation during late spontaneous recovery. All these have increased the risk of nerve torsion and reduced reinnervation activity.24 Magnetic resonance and high‐resolution ultrasound imaging are established tools for detecting structural changes in the involved nerves. Surgical procedures such as neurolysis, nerve grafts, and nerve transfers are shown to have an advantage over conservative treatment in terms of nerve constriction and torsion management.25
Conclusions
The present case is a rare incidence of Brucella infection as a possible trigger for Neuralgic Amyotrophy and is thought to be the first such case report in China.The possibility of NA should be closely monitored for when a patient with brucellosis in those presenting with acute onset of severe pain with rapid multifocal weakness and atrophy in shoulder girdle muscle, multifocal axonal loss from peripheral origin and numerous denervation signs on electromyography, multifocal hyperintense signal area in MRI neuroimaging of the brachial plexus and ineffective antibiotic treatment. Based on our belief that an immune-mediated process of Brucella infection is likely a trigger for NA, when persisting symptoms after anti-Brucella treatment, treatment trials with corticosteroids and immunoglobulin should be attempted at the early stage.
Abbreviations
NA, Neuralgic Amyotrophy; GBS, Guillain-Barre Syndrome; RBPT, Rose Bengal Plate Test; SAT, Serum Agglutination Test; WBC, White Blood Cell; AST, Aspartate aminotransferase; PIT, Platelet; GGT, Glutamine transpeptidase; LAP, Leucine arylamidase; GLDH, Glutamate dehydrogenase; ALP, Alkaline phosphatase; EMG, Electromyography; MCV, Motor Conduction Velocity; MRI, Magnetic Resonance Imaging; MNAP, Median Nerve Action Potential; CMAP, Compound Muscle Action Potential; Lat, Latency; Amp, Amplitudes; MU, Motor Unit.
Patient Consent and Ethics Statement
The study was carried out in accordance with the recommendations of the Ethics Committee of the China-Japan Union Hospital of Jilin University. A written permission for the use of patient data for publication of this cases report and accompanying images was obtained.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study did not receive any funding.
Disclosure
The authors declare that they have no competing interests for this study.
References
1. Ijspeert J, Janssen RMJ, van Alfen N. Neuralgic amyotrophy. Curr Opin Neurol. 2021;34(5):605–612. doi:10.1097/WCO.0000000000000968
2. van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol. 2011;7(6):315–322. doi:10.1038/nrneurol.2011.62
3. van Alfen N, van Engelen BGM. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129:438–450. doi:10.1093/brain/awh722
4. Stek CJ, van Eijk JJJ, Jacobs BC, et al. Neuralgic amyotrophy associated with Bartonella henselae infection. J Neurol Neurosurg Psychiatry. 2011;82(6):707–708. doi:10.1136/jnnp.2009.191940
5. Ismail II, Abdelnabi EA, Al-Hashel JY, et al. Neuralgic amyotrophy associated with COVID-19 infection: a case report and review of the literature. Neurol Sci. 2021;42(6):2161–2165. doi:10.1007/s10072-021-05197-z
6. Bahemuka M, Shemena AR, Panayiotopoulos CP, et al. Neurological syndromes of brucellosis. J Neurol Neurosurg Psychiatry. 1988;51(8):1017–1021. doi:10.1136/jnnp.51.8.1017
7. Ara JR, Oliveros A. Neuralgic amyotrophy of the shoulder associated with systemic brucellosis. Med Clin. 1992;99(20):794–795.
8. Yagupsky P, Morata P, Colmenero JD. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. 2020;33(1):e00073.
9. Buzgan T, Karahocagil MK, Irmak H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis. 2010;14(6):E469–E478. doi:10.1016/j.ijid.2009.06.031
10. van Eijk JJJ, van Alfen N, Berrevoets M, et al. Evaluation of prednisolone treatment in the acute phase of neuralgic amyotrophy: an observational study. J Neurol Neurosurg Psychiatry. 2009;80(10):1120–1124. doi:10.1136/jnnp.2008.163386
11. Zheng R, Xie S, Lu X, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int. 2018;2018:1–10. doi:10.1155/2018/5712920
12. Shanina E, Liao B, Smith RG. Brachial plexopathies: update on treatment. Curr Treat Options Neurol. 2019;21(5):5. doi:10.1007/s11940-019-0562-5
13. Stutz CM. Neuralgic amyotrophy: parsonage-turner syndrome. J Hand Surg Am. 2010;35A(12):2104–2106. doi:10.1016/j.jhsa.2010.09.010
14. Yang LJS. Neonatal brachial plexus palsy-Management and prognostic factors. Semin Perinatol. 2014;38(4):222–234. doi:10.1053/j.semperi.2014.04.009
15. Kim TU, Chang MC. Neuralgic amyotrophy: an underrecognized entity. J Int Med Res. 2021;49(4):030006052110065. doi:10.1177/03000605211006542
16. Seror P. Neuralgic amyotrophy an update. Joint Bone Spine. 2017;84(2):153–158. doi:10.1016/j.jbspin.2016.03.005
17. Sneag DB, Saltzman EB, Meister DW, et al. MRI Bullseye sign: an indicator of peripheral nerve constriction in parsonage-turner syndrome. Muscle Nerve. 2017;56(1):99–106. doi:10.1002/mus.25480
18. Lee JS, Kim YT, Kim JS, et al. The clinical manifestations and outcomes of neuralgic amyotrophy. Neurol Asia. 2017;22(1):9–13.
19. Ang CW, Jacobs BC, Laman JD. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25(2):61–66. doi:10.1016/j.it.2003.12.004
20. Watanabe K, Kim S, Nishiguchi M, et al. Brucella melitensis infection associated with Guillain-Barré syndrome through molecular mimicry of host structures. FEMS Immunol Med Microbiol. 2005;45(2):121–127. doi:10.1016/j.femsim.2005.03.001
21. Suarez GA, Giannini C, Bosch EP, et al. Immune brachial plexus neuropathy: suggestive evidence for an inflammatory-immune pathogenesis. Neurology. 1996;46(2):559–561. doi:10.1212/WNL.46.2.559
22. Skendros P, Pappas G, Boura P. Cell-mediated immunity in human brucellosis. Microbes Infect. 2011;13(2):134–142. doi:10.1016/j.micinf.2010.10.015
23. Johnson NE, Petraglia AL, Huang JH, et al. Rapid resolution of severe neuralgic amyotrophy after treatment with corticosteroids and intravenous immunoglobulin. Muscle Nerve. 2011;44(2):304–305. doi:10.1002/mus.22100
24. Lieba-Samal D, Jengojan S, Kasprian G, et al. Neuroimaging of classic neuralgic amyotrophy. Muscle Nerve. 2016;54(6):1079–1085. doi:10.1002/mus.25147
25. Gstoettner C, Mayer JA, Rassam S, et al. Neuralgic amyotrophy: a paradigm shift in diagnosis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):879–888. doi:10.1136/jnnp-2020-323164
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.