Back to Journals » International Medical Case Reports Journal » Volume 16
A Rare Case of Hair Dye Induced Oral Lichenoid Reaction
Authors Aboalela A
Received 22 March 2023
Accepted for publication 1 June 2023
Published 6 June 2023 Volume 2023:16 Pages 345—350
DOI https://doi.org/10.2147/IMCRJ.S410639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Ali Aboalela1– 3
1Maxillofacial Surgery and Diagnostic Sciences Department, College of Dentistry, King Saud bin Abdulaziz University for Health Sciences (KSAU- HS), Riyadh, Saudi Arabia; 2King Abdullah International Medical Research Center, Riyadh, Saudi Arabia; 3Dental Services, Ministry of the National Guard - Health Affairs, Riyadh, Saudi Arabia
Correspondence: Ali Aboalela, Maxillofacial Surgery and Diagnostic Sciences Department, College of Dentistry, King Saud bin Abdulaziz University for Health Sciences (KSAU-HS), P.O. Box.22490, Riyadh, 11426, Kingdom of Saudi Arabia, Tel +966 11 4299999 Ext.95879, Email [email protected]; [email protected]
Abstract: Oral lichenoid lesions or reactions (OLLs/OLRs), which are clinical and histological contemporaries of the traditional oral lichen planus (OLP), had already garnered a great deal of attention in the literature. In contrast to idiopathic OLP, OLLs frequently have a definite, recognizable initiating component. Although a cursory clinical and histological evaluation of lesions frequently demonstrates numerous similarities with OLP, relatively new data has demonstrated that distinct features exist and serve as the foundation for the majority of categories. Although many systemic pharmaceuticals can lead to end oral lichenoid reactions, medications for diabetes, hypertension, nonsteroidal anti-inflammatory, antimalarial, and antifungal treatments are frequently blamed. Oral drugs, metallic dental restorations, acrylates, composite resins, glass ionomer cement, cinnamates, flavorings, and other chemical substances have all been associated when in direct contact. The objective of the case report is to elaborate the correlation between the oral lichenoid reaction and the use of hair dye. The incident under consideration is significant because the majority of past reports of allergic reactions to hair dye involved the face and scalp rather than the oral cavity. This report recommends that oral physicians inquire about the patient’s use of cosmetics during history-taking whenever dealing with abrupt inflammatory responses in the orofacial area in order to diagnose and treat lesions more efficiently.
Keywords: drug association, malignant transformation, oral lichenoid lesions, oral lichenoid reactions
Introduction
Numerous hair dyes have been in use since the beginning of time.1 Several types of hair dyes include nonoxidative (temporary and semi-permanent) and oxidative (semi-permanent and permanent) synthetic dyes as well as natural colors like henna and metallic dyes made from silver salts and lead.2 Vegetable hair dyes seldom cause contact allergies, whereas paraphenylenediamine (PPD), a well-known allergen linked to allergic contact dermatitis from hair dyes, has frequently been associated3–5 It has been a main component for many years6 and is the preferred component in many hair dye recipes because of its ability to provide a more durable accentuated black or natural color.5
This article is a report on a case of hypersensitivity reaction resulting from the change in hair dye used to dye a beard in an adult Saudi patient. In this report, we describe a Saudi male patient presenting with oral hypersensitivity reaction to a hair dye that resolved following discontinuation of the dye.
Case Report
In the present case, a 66-year-old Saudi man was referred to the KAMC in August 2016 to Oral medicine clinic with recurrent swelling with diffuse pigmentation and occasional ulceration in the lower lip area that occasionally appeared and dissipated without the need for any medical or surgical intervention. The patient’s medical history revealed type II diabetes from over two decades. A recent history of dyeing the beard with a commercial hair dye was noted. The swelling had enlarged over the next few days and subsided after a week, the swelling recurred after two weeks and subsided without any intervention which was suggestive towards a mucocele, the patient gave a similar episode of pigmentation and swelling on the subsequent application of the hair dye. The patient was advised to discontinue the hair dye application to monitor the recovery. However, the patient refused to comply with the instructions given and instead changed the brand of the hair dye.
The patient further reported scaling of the lips and sporadic burning sensation. The lower left alveolar ridge and labial mucosa had an asymptomatic dark grey pigmentation of 2–3 cm associated with mild erythema and ulceration (Figure 1) without any changes in its size or shape. Reassurance followed by topical triamcinolone 0.1% orabase application for the labial mucosa was prescribed and the patient was recalled for follow up after two weeks. At the two-week follow up, the lip appeared the same, but there was increased scaling and itching and there was absence of swelling without sign of lymph node involvement. Topical tacrolimus was prescribed for the lips along with the previous orabase. At the follow-up visit after one month patient stated that the topical tacrolimus treatment had 20% improvement for the itching and scaling, but had decided to stop using it on his own since the symptoms did not completely resolve. The itching and scaling of the lips were considered to be an allergic reaction to the dye suggestive of contact mucositis/lichenoid mucositis. Furthermore, the recurrence of similar lesions on his lower lip and labial mucosa after three month follow-up necessitated a biopsy; an incisional biopsy was planned for the patient to identify the cause of such reaction/lesions. The patient was also requested to discontinue any hair dyes until follow-up.
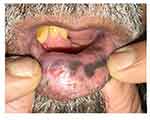 |
Figure 1 Clinical photograph showing gray pigmentation on the lower labial mucosa with ulceration. |
Biopsy revealed eczematous dermatitis with mild edema and neutrophilic parakeratosis. Parakeratinized stratified squamous epithelium with acanthosis, mild spongiosis and leukocyte exocytosis with degeneration of the basal cells. Epithelium shows focal spongiotic pustules and intraepithelial micro abscesses (Figure 2). Diffuse intense chronic inflammatory cell infiltrate consisting of lymphocytes, plasma cells and eosinophils is present at the juxta epithelial as well as deeper connective tissue. Peri and para-vascular inflammatory cells are also noted within the deeper connective tissue (Figure 3). Image was suggestive of allergic reaction contact dermatitis at the 1-week follow-up and topical tacrolimus was prescribed. 90% improvement with minimal itching was seen within 2 weeks of continued avoidance of dye and topical tacrolimus application confirming the allergic reaction to the dye. Although the patient was scheduled for a follow up after one month, the patient attended only after 2 years revealing complete resolution of symptoms and most of the pigmentation (Figure 4).
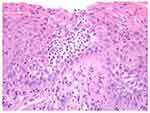 |
Figure 2 Hematoxylin and Eosin Stain, magnification X200, showing inflamed and ulcerated mucosa with focal thickening of the basement membrane, spongiotic pustules and intraepithelial microabscesses. |
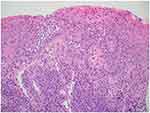 |
Figure 3 Hematoxylin and Eosin Stain, magnification X100 showing chronic inflammatory cell infiltrate present at the juxta epithelial and deeper connective tissue. |
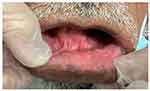 |
Figure 4 Clinical photograph showing complete resolution of symptoms and pigmentation after discontinuing the dye. |
Discussion
Generally, oral lichenoid lesions can be identified by visual inspection alone without the need for further testing; however, in most cases, additional investigations are required. Oral lichenoid lesions often present overlapping clinical and histopathological features and diagnosis is frequently challenging. A comprehensive clinical assessment with detailed history by a multidisciplinary group of specialists may be warranted to further investigate skin, nail, scalp, genital, esophageal, laryngeal, and conjunctival involvement. In this case, oral lichenoid reaction and diabetes did not appear to be associated, as the patient had been on the same medication for approximately 20 years and symptoms appeared around the time of dye use. Additionally, resolution of all signs and symptoms occurred during the continued use of the same anti-diabetic medications.
A number of oral lichenoid lesions affect the oral mucosa, much like the skin counterpart, and include oral lichenoid drug reactions; oral lichenoid contact lesion; oral lichen planus; lichen planus pemphigoid; lichen sclerosis; oral lichenoid lesions of graft-versus-host disease; oral lesions of systemic lupus erythematosus; oral discoid lupus erythematosus; erythema multiforme; paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome; lichenoid and granulomatous stomatitis; and chronic ulcerative stomatitis. Traditionally, the finding of oral lichenoid response/reactions depends on clinical and histological correlation, yet in a few occurrences this approach falls short of delivering a trustworthy diagnosis. Therefore, utilizing molecular methods might improve our capacity to distinguish between oral lichenoid reactions.
Hair dyes are frequently used cosmetics by both young and old people, hair dye dermatitis is an established well substantiated disorder.7 The frequency of side effects related to hair dye has increased as a result of an expanding hair coloring trend.8 Allergic reactions have been increased globally, but especially in Asia.9 Even though sporadic cases of hair dye dermatitis were seen in Iraq, they were increasing over time especially since 2016.10 Unfortunately, because there is a wide variation in the features and location of a reaction it is difficult to make a diagnosis, especially if the patient’s usage of hair dye is not specifically mentioned. The face is the area most frequently affected by hair dye-related dermatitis, followed by the scalp area.10 The scalp is relatively spared, save for the edge, because it has thick skin, which makes it difficult for the dye to penetrate and produce sensitization. Due to the fact that the patient’s initial complaint does not affect the scalp, the diagnosis may be readily missed. Oral lichenoid lesions usually appear as solitary lesions unlike oral lichen planus and the area of occurrence is peculiar.9
In the scientific literature, there is ample evidence of a similarity between contact dermatitis and lichenoid drug reactions; nevertheless, the prevalence and etiology of oral lichenoid drug reactions are debatable.11 It can be challenging to differentiate oral lichenoid reactions (OLDRs) from immune-mediated oral lichen planus (OLP) because oral lichenoid lesions (OLLs) are histologically and clinically similar to OLP. The existence of neutrophils, eosinophils, and plasma cells in the deeper layers of drug-induced lesions with increased apoptotic keratinocytes is proposed by evidence. Histopathologically, it is similar to oral lichen planus in that it exhibits marked epithelial acanthosis and elongated rete ridges with a mix of perivascular inflammatory response and inflammatory cell infiltrates.12,13 The histologic picture in this case is confirmatory and similar to that described in the literature,12,13 with sub basal chronic inflammatory cells trying to infiltrate the hyperplastic epithelium and classical rete ridges (Figure 3).
Clinical manifestations of hair dye dermatitis include prurigo-like lesions, allergic contact dermatitis, photo contact dermatitis, contact urticaria, contact leukoderma, contact anaphylaxis, photosensitivity, and airborne contact dermatitis are all instances of allergic contact dermatitis. Non-eczematous phenotypic traits of adverse reactions to hair dye encompass lymphomatoid contact dermatitis, dysmorphic syndrome, eye lens toxicity, renal impairment, proteinuria, hematuria, hypertension, and bronchospasm. Hair dyes with hydrogen peroxide may cause structural hair shaft damage, loss of eyelashes might even occur by using PPD-containing mascara. Another cause of concern is the correlation between the use of hair dyes and cancer.7 PPD is the most common cause of hair dye dermatitis,4,5 and PPD can cross-react with local ester anesthetics, rubber Toluene −2, 5- diamine.14 The incisional biopsy of the lesion revealed a lichenoid-like reaction pathological picture which is similar to the appearance in the Iraqi population studied by Sharquie et al who reported a hypersensitivity reaction to chemical components in the hair dye.10
It is still unclear what exactly causes haptens to be associated with PPD sensitivity. The metabolism in the skin, in particular the p-benzoquinone, is thought to be the cause of its cross-reactivity and allergenicity. Another theory is that the allergenicity of Bandrowski’s base, a form of PPD generated upon exposure.15–17 It has also been proposed that a genetic variability in the cutaneous metabolism of PPD explains why only a small number of people who apply hair dyes well have contact sensitivity or varied allergic reactions.18 According to reports, the prevalence of PPD dermatitis was 4.3% in Asia, 4% in Europe, and 6.2% in North America.7 Hair dye dermatitis is diagnosed primarily through history and clinical examination.19 An epicutaneous patch test is the gold standard test for confirming hair dye contact allergy.17,18 Open testing is recommended by hair dye manufacturers to test for allergic reactions 48 hours prior to hair dye applications.20,21
Conclusion
The case at discussion is noteworthy because the majority of earlier reports of allergic responses to hair dye involved the face and scalp rather than the oral cavity. In order to diagnose and treat the lesion more quickly, this report recommends the clinician to inquire about the patient’s usage of cosmetics during history-taking when dealing with abrupt inflammatory responses in the orofacial region.
Ethical Approval
Approval from a formally constituted review board from (institutional review board/ethics committee) College of Dentistry, King Saud bin Abdulaziz university of Health Sciences. Attached to King Abdullah International Medical Research was obtained.
Consent for Publication
Written informed consent was provided by the patients to have the case details and any accompanying images published.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Platzek T. Risk from exposure to arylamines from consumer products and hair dyes. Front Biosci. 2010;2(3):1169–1183. doi:10.2741/e177
2. França SAD, Dario MF, Esteves VB, Baby AR, Velasco MVR. Types of hair dye and their mechanisms of action. Cosmet. 2015;2(2):110–126. doi:10.3390/cosmetics2020110
3. Thami GP, Kaur S, Kanwar AJ. ACC to henna. Allergy. 2001;56:1013–1014. doi:10.1034/j.1398-9995.2001.00376.x
4. Meyer A, Fischer K. Oxidative transformation processes and products of PPD and (PTD)—a review. Environ Sci Eur. 2015;27(1):11. doi:10.1186/s12302-015-0044-7
5. Mukkanna KS, Stone NM, Ingram JR. Para-phenylenediamine allergy: allergy: current perspectives on diagnosis and management. J Asthma Allergy. 2017;10:9–15. doi:10.2147/JAA.S90265
6. Hofmann AW. Organische Basen. Jahresberichte ueber die Fortschritte der Chemie. Annu Rep Prog Chem. 1863;3:422.
7. Handa S, Mahajan R, De D. Contact dermatitis to hair dye: an update. Indian J Dermatol Venereol Leprol. 2012;78(5):583–590. doi:10.4103/0378-6323.100556
8. Hamann D1, Yazar K, Hamann CR, Thyssen JP, Lidén C. p-Phenylenediamine and other allergens in hair dye products in the United States: a consumer exposure study. Contact Dermatitis. 2014;70(40):213–218. doi:10.1111/cod.12164
9. Serrano Sánchez P, Bagán JV, Jiménez-Soriano SG. Drug-induced oral lichenoid reactions: a literature review. J Clin Exp Dent. 2010;2(2):e71–e75. doi:10.4317/jced.2.e71
10. Sharquie KE, Noaimi AA, Hasan RL. Outbreak of hair dye dermatitis with imitating tendency. Am J Clin Dermatol Venereol. 2020;9(1):1–5.
11. Yuan A, Woo SB. Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(1):35–47. doi:10.1016/j.oooo.2014.09.009
12. Cheng YSL, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(3):332–354. doi:10.1016/j.oooo.2016.05.004
13. Thornhill MH, Sankar V, Xu XJ, et al. The role of histopathological characteristics in distinguishing amalgam‐associated oral lichenoid reactions and oral lichen planus. J Oral Pathol Med. 2016;35(4):233–240. doi:10.1111/j.1600-0714.2006.00406.x
14. Singh V, Ali M, Upadhyay S. Study of coloring effect of herbal hair formulations on graying hair. Pharmacognosy Res. 2015;7(3):259–262. doi:10.4103/0974-8490.157976
15. Thomas R, White IR, McFadden JP, Banerjee P. Positive relationship-intensity of response to on patch testing and cross-reactions with related allergens. Contact Dermatitis. 2014;71(2):98–101. doi:10.1111/cod.12255
16. Castanedo-Tardan MP, Zug KA. Allergic contac dermatitis. In: Goldsmith LA, Kataz SI, Gilchrest BA, Paller SA, Leffell DJ, Wolf K, editors. Fitzpatrick’s Dermatology in General Medicine 8th Ed. NewYork: McGraw Hill; 2012:155–156.
17. McFadden JP, Yeo L, White JL. Clinical and experimental aspects of allergic contact dermatitis to (PPD). Clin Dermatol. 2011;29(3):316–324. doi:10.1016/j.clindermatol.2010.11.011
18. Ho SG, White IR, Rycroft RJ, McFadden JP. A new approach to patch testing patients with (PPD) allergy secondary to temporary black henna tattoos. Contact Dermatitis. 2004;51:213–214. doi:10.1111/j.0105-1873.2004.0424d.x
19. Draelos ZD. Cosmetics and cosmeceuticals. In: Bolognia JL, Jorizo JL, Schaffer JV, editors. Textbook of Dermatology, 3rd ed. Vol. 2386. China: Elsevier; 2012.
20. Krasteva M, Cottin M, Cristaudo A, et al. Sensitivity and specificity of the consumer open skin allergy test as a method of prediction of contact dermatitis to hair dyes. Eur J Dermatol. 2005;15(1):18–25.
21. Krasteva M, Cristaudo A, Hall B, et al. Contact sensitivity to hair dyes can be detected by the consumer open test. Eur J Dermatol. 2002;12(4):322–326.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
