Back to Journals » Infection and Drug Resistance » Volume 15
A Rare Case of Co-Infection with Nocardia farcinica, Pneumocystis jirovecii, and Aspergillus fumigatus Due to Tooth Extraction in a Mildly Immunosuppressed Patient
Authors Jinlin G , Shaohui S, Wenjun Z, Xinfeng C
Received 18 June 2022
Accepted for publication 20 August 2022
Published 25 August 2022 Volume 2022:15 Pages 4853—4857
DOI https://doi.org/10.2147/IDR.S379005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guo Jinlin,1,* Song Shaohui,2,* Zhang Wenjun,3 Cai Xinfeng4
1Department of Pharmacy, Shanxi Provincial People’s Hospital, Taiyuan, People’s Republic of China; 2Department of Pharmacy, The Maternal and Child Health Hospital of Dadukou District of Chongqing City, Chongqing, People’s Republic of China; 3Department of Pharmacy, Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 4Department of Pharmacy, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cai Xinfeng, Shanxi Province Cancer Hospital, Taiyuan, Shanxi, 030012, People’s Republic of China, Tel +86-18835167718, Email [email protected]
Abstract: We report a case of co-infection with Nocardia farcinica, Pneumocystis jirovecii, and Aspergillus fumigatus due to tooth extraction in a mildly immunosuppressed patient. This patient did not respond well to a meropenem-based regimen, and the number of lesions was significantly reduced after switching to imipenem. The patient’s trough concentration was insufficient when using conventional doses of voriconazole for the treatment of pulmonary aspergillosis. After adding omeprazole, the concentration reached standard levels and symptoms improved. The patient eventually made a full recovery.
Keywords: Aspergillus fumigatus, interaction, intracranial infection, Nocardia farcinica, Pneumocystis jirovecii, voriconazole
Plain Language Summary
- Nocardia farcinica, Pneumocystis jirovecii, and Aspergillus fumigatus are opportunistic pathogens, and co-infection of these three species is very rare.
- We report a case of co-infection with Nocardia farcinica, Pneumocystis jirovecii, and Aspergillus fumigatus in an immunosuppressed patient.
- This patient did not respond well to a meropenem-based regimen, and the number of lesions was significantly reduced after switching to imipenem.
- For the treatment of N. farcinica, especially for intracranial infection, imipenem is superior to meropenem.
- During treatment with voriconazole, if the trough concentration of voriconazole does not reach the target, the concentration can be increased by adding omeprazole.
Introduction
Immunosuppressed patients are prone to opportunistic infections.1 Nocardia farcinica, Pneumocystis jirovecii, and Aspergillus fumigatus are opportunistic pathogens, and their risk factors include high-dose glucocorticoids or immunosuppressants, AIDS, solid organ transplantation, and hematological tumors.2–4 The mortality rate of such infections is high, and clinical treatment is a great challenge. Co-infection of these three species has not been reported before. We report a case of successful treatment of intracranial infection with N. farcinica combined with pulmonary infection with P. jirovecii and A. fumigatus.
Case Presentation
A 62-year-old male took dexamethasone tablets (2.25 mg three times per day) and ornidazole tablets (0.5g two times per day) orally after tooth extraction. Ten days later, he developed fever (body temperature of 39°C) accompanied by chills, headache, bloating, tinnitus, and hearing loss. Treatment at the local hospital was not effective, and he was transferred to our hospital three days later. On admission, the patient’s body temperature was 38.6°C, blood pressure was 134/74 mmHg, respiration was 16 beats/min, heart rate was 75 beats/min, and he was conscious. Relevant examinations were as follows: white blood cell count of 4.40×109/L, neutrophil percentage of 89.8%, lymphocyte count of 0.17×109/L, CD4+ lymphocyte count of 32/mm3, C-reactive protein level of 14.38 mg/L, creatinine level of 37.05 umol/L, pH of 7.399, procalcitonin level of 0.123 ng/mL, and 1,3-β-
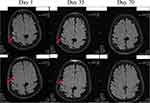 |
Figure 2 Magnetic resonance imaging scan of the patient. |
Discussion
To the best of our knowledge, this is the first report of an immunocompromised patient with N. farcinica, P. jirovecii, and A. fumigatus co-infection. The patient presented with headache and hearing loss, and multiple lesions were observed on head MRI, which is indicative of intracranial infection. The number of sequences from N. farcinica in the patient’s CSF was very high and CSF examination showed N. farcinica infection in the patient’s brain. The patient’s CSF examination was consistent with purulent meningitis.5
The patient had respiratory dysfunction and low immune function; the CD4+ lymphocyte count was only 32/mm3, ground-glass shadows could be seen on chest CT, sequences from P. jirovecii were detected in the patient’s blood and 1,3-β-
Using the search terms N. farcinica and P. jirovecii on PubMed, the co-infection of Nocardia and P. jirovecii was found to be very rare and only one report describe this kind of infection and the patient was on SMZ based treatment.10 SMZ is the main treatment for Nocardia and P. jirovecii. Amikacin, imipenem, meropenem, linezolid, and amoxicillin-clavulanate potassium are all active against N. farcinica. If dissemination or central nervous system infection occurs, at least two drugs should be selected for treatment.11 However, amikacin cannot reach an effective concentration intracranially, but imipenem is clinically effective and its activity is stronger than that of meropenem and linezolid can achieve a high intracranial concentration and can be used for severe infections.12 Imipenem can maintain higher concentrations intracranially than meropenem in cerebrospinal fluid and is four times more active against Nocardia.13,14 The patient’s initial treatment with a meropenem-based regimen for one month was ineffective, and a month after imipenem was substituted, the lesions were significantly reduced, proving that imipenem is superior to meropenem in the treatment of intracranial infection with N. farcinica.
On the 18th day, the patient’s sputum culture was positive for A. fumigatus, and CT showed a new lesion. The doctor considered fungal infection and initiated voriconazole for treatment. However, during treatment, the patient occasionally had blood in the sputum, and chest CT showed that the infection was still progressing. The voriconazole trough concentration was 0.8 mg/L. According to guidelines and previous reports, the trough concentration of voriconazole should be maintained between 1 and 6 mg/L.15,16 Therefore, the poor antifungal effect observed in patients is likely related to the insufficient concentration of voriconazole, but voriconazole is very expensive and the treatment course for fungal infections is relatively long so patients may not be able to afford higher doses. The label of voriconazole states that CYP450 inhibitors such as omeprazole and esomeprazole will lead to an increase in voriconazole concentration.17 Therefore, we added omeprazole to the patient’s treatment regimen. After three days, the patient’s voriconazole trough concentration reached the treatment standard, and the patient’s symptoms gradually improved. After about two months of voriconazole treatment, it was discontinued. Therefore, we believe that for patients with insufficient voriconazole concentrations at conventional doses, the voriconazole concentration can be increased by adding omeprazole.
There were some limitations in this study. Our microbiology laboratory failed to culture Nocardia and P. jirovecii, and we were unable to perform drug susceptibility testing for Aspergillus. Therefore, the drug susceptibility to these three pathogens can only be based on previous literature reports. Considering the problem of fungal resistance, drug susceptibility testing should be performed before using triazoles and the treatment strategy with voriconazole is only applicable in MIC≤1 ug/mL for Aspergillus.
Conclusion
N. farcinica, P. jirovecii and A. fumigatus are opportunistic pathogens, and co-infection of these three species is very rare. These pathogens are usually associated with strong, long-lasting immunosuppressive regimens, but the tooth extraction-associated incident described in this report highlights the need to pay attention even to such inconspicuous cases. For the treatment of N. farcinica, especially for intracranial infection, imipenem is superior to meropenem. During treatment with voriconazole, if the trough concentration of voriconazole does not reach the target, the concentration can be increased by adding omeprazole.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available since the patient’s medical records and data are private, but can be made available from the corresponding author on reasonable request under the consent from the patient.
Compliance with Ethics Guidelines
This study was conducted following the legal requirements and the Declaration of Helsinki and its subsequent amendments. Informed consent for publication was obtained from the patient. According to the hospital protocol, no formal ethics approval was required for this study.
Acknowledgments
We would like to thank the patient for their approval of this paper.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Wang JGL, Xu YN. Risk factor analysis of respiratory tract infection in elderly patients in a tertiary grade A hospital cadre ward. Med J Chin PLA. 2020;38(8):41–43.
2. Abreu C, Rocha-Pereira N, Sarmento A, Magro F. Nocardia infections among immunomodulated inflammatory bowel disease patients: a review. World J Gastroenterol. 2015;21(21):6491–6498. doi:10.3748/wjg.v21.i21.6491
3. Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi:10.1093/cid/ciw326
4. Lagrou K, Chen S, Masur H, et al. Pneumocystis jirovecii disease: basis for the revised EORTC/MSGERC invasive fungal disease definitions in individuals without human immunodeficiency virus. Clin Infect Dis. 2021;72(Suppl 2):S114–S120. doi:10.1093/cid/ciaa1805
5. Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi:10.1093/cid/ciw861
6. Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19(1):39–49. doi:10.1111/j.1469-0691.2011.03760.x
7. Theel ES, Jespersen DJ, Iqbal S, et al. Detection of (1, 3)-beta-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia. 2013;175(1–2):33–41. doi:10.1007/s11046-012-9579-y
8. Morjaria S, Frame J, Franco-Garcia A, Geyer A, Kamboj M, Babady NE. Clinical performance of (1,3) Beta-D glucan for the diagnosis of pneumocystis pneumonia (PCP) in cancer patients tested with PCP polymerase chain reaction. Clin Infect Dis. 2019;69(8):1303–1309. doi:10.1093/cid/ciy1072
9. Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis. 2011;53(2):197–202. doi:10.1093/cid/cir335
10. Hou J, Cao J, Tan P, Yu Y. Pneumocystis jiroveci pneumonia, Nocardia brasiliensis, and Mycobacterium tuberculosis co-infection in a myasthenia gravis patient: a case report. Medicine. 2021;100(1):e24245. doi:10.1097/MD.0000000000024245
11. Restrepo A, Clark NM. Infectious diseases community of practice of the American Society of T. Nocardia infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant. 2019;33(9):e13509. doi:10.1111/ctr.13509
12. John G, Bartlett PGA, Paul A. The Johns Hopkins ABX Guide. Beijing: Scientific and Technical Documentation Press; 2012.
13. Cercenado E, Marin M, Sanchez-Martinez M, Cuevas O, Martinez-Alarcon J, Bouza E. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob Agents Chemother. 2007;51(3):1102–1104. doi:10.1128/AAC.01102-06
14. David N, Gilbert HFC, Michael S. The Sanford Guide to Antimicrobial Therapy 2020.
15. Park WB, Kim NH, Kim KH, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55(8):1080–1087. doi:10.1093/cid/cis599
16. John J, Loo A, Mazur S, Walsh TJ. Therapeutic drug monitoring of systemic antifungal agents: a pragmatic approach for adult and pediatric patients. Expert Opin Drug Metab Toxicol. 2019;15(11):881–895. doi:10.1080/17425255.2019.1671971
17. Chen K, Zhang X, Ke X, Du G, Yang K, Zhai S. Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese Pharmacological Society. Ther Drug Monit. 2018;40(6):663–674. doi:10.1097/FTD.0000000000000561
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.