Back to Journals » International Journal of Women's Health » Volume 14
A Rare Case of Cervical Squamous Cell Carcinoma Concurrent with Sinonasal Inverted Papilloma: A Case Report
Authors Hou XY, Peng CR, Zhang GN, Wang DF
Received 28 June 2022
Accepted for publication 17 November 2022
Published 29 November 2022 Volume 2022:14 Pages 1657—1666
DOI https://doi.org/10.2147/IJWH.S380385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Xiao Yu Hou,1 Chun Rong Peng,2 Guo Nan Zhang,2 Deng Feng Wang2
1School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 2Department of Gynecologic Oncology, Sichuan Cancer Hospital & Institute, The Affiliated Cancer Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
Correspondence: Deng Feng Wang, Department of Gynecologic Oncology, Sichuan Cancer Hospital & Institute, The Affiliated Cancer Hospital, School of Medicine, University of Electronic Science and Technology of China, No. 55, Section 4, South Renmin Road, Chengdu, 610041, People’s Republic of China, Tel +86 15982222707, Fax +86 28 85420116, Email [email protected]
Introduction: Cervical cancer is the fourth most common malignancy in women worldwide, and sinonasal inverted papilloma (SIP) is a rare benign sinus tumor with characteristics including a destructive growth pattern, high recurrence rate, and common malignant transformation. Cervical squamous cell carcinoma (SCC) combined with SIP has not been reported thus far.
Case Presentation: A 55-year-old woman was diagnosed with cervical SCC in our center and treated with concurrent radiochemotherapy. During the follow-up period after the completion of cervical cancer treatment, the progression of cervical squamous cell carcinoma was first considered because the squamous cell carcinoma antigen (SCCA) levels remained high and slowly increased. However, SIP was found after a detailed investigation. The SCCA levels returned to normal after surgery. Two months after the surgery, because SCCA slowly increased again, it was found that the SIP recurred. After additional surgical treatment, the SCCA level returned to normal.
Discussion and Conclusion: First, SCCA is an important indicator for monitoring changes in cervical SCC. When the changes in SCCA levels are inconsistent with the prognosis of cervical SCC, we should be vigilant about considering the possibility of other diseases existing at other sites in the body, which might lead to the earlier detection and treatment of SIP. Second, We recommended that SCCA be used as a routine monitoring index for SIP. If available, SCCA1 and SCCA2 should be evaluated to provide a more detailed assessment. Finally, for a high recurrence rate of SIP, anti-HPV treatment might be considered to reduce the risk of recurrence.
Keywords: cervical squamous cell carcinoma, inverted papilloma, squamous cell carcinoma antigen, human papilloma virus
Introduction
Cervical cancer is the fourth most common malignancy in women worldwide, with 84% occurring in developing countries, and it is the most common malignancy of the female reproductive system in China.1 In 2020, 604,000 new cases of cervical cancer were reported globally, with 42,000 deaths.2 There are several different histological types of cervical cancer, including cervical squamous cell carcinoma (SCC), cervical adenocarcinoma, cervical adenosquamous carcinoma, and other rare types, of which SCC accounts for about 75–80%.3 Surgery is the primary treatment for early-stage cervical cancer, while concurrent radiochemotherapy is the preferred treatment for middle and advanced stages (International Federation of Gynecology and Obstetrics (FIGO) 2018 stages IIB-IVA).4
Sinonasal papilloma (SP), previously known as Schneiderian papilloma, includes three major subtypes: inverted, oncocytic, and exophytic papillomas.5 Sinonasal inverted papilloma (SIP) is the most common subtype and is a rare benign sinus tumor with an incidence of 0.74–1.5/100,000 people per year.6 SIP originates from the respiratory mucosa that differentiates from ectoderm and accounts for 0.4–4.7% of all nasal cavity tumors.7 SIP tends to occur in the age range of 50–70 years and has a high malignant transformation rate (13.64%) and recurrence rate (34.09%).8,9 Clinical manifestations of this disease include unilateral nasal congestion, epistaxis, headache, sinusitis, loss of smell, otitis media, vertigo, hearing loss, diplopia, periorbital swelling, and others.10 In addition, 90% of the cases are unilateral.11 Cervical SCC combined with SIP has not been reported previously. Therefore, we have described a case treated in our hospital.
Case Presentation
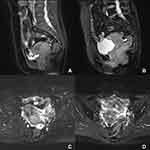
A female 55-year-old patient was admitted to our hospital in October 2019 due to “irregular vaginal bleeding for two months after menopause.” A gynecological physical examination revealed a normal vulva and unobstructed vagina, but the vaginal fornix was not visible. The cervix was nodular and cauliflower-shaped, approximately 5 cm in diameter, and positive for blood on palpation. The bilateral parauterine tissues were firm, shortened, and did not reach the lateral pelvic wall. No obvious abnormalities were observed in the uterine body and bilateral adnexal areas. Before admission, cervical SCC was detected via a cervical biopsy in another hospital. The pathology consultation in our hospital indicated the presence of a medium, poorly differentiated cervical SCC. After admission, additional relevant examinations and laboratory tests were completed. The serum carcinoembryonic antigen (CEA) was 9.86 ng/mL (normal range 0–5 ng/mL) and the squamous cell carcinoma antigen (SCCA) was 19.60 ng/mL (normal range 0–1.8ng/mL). Enhanced magnetic resonance imaging (MRI) of the entire abdomen revealed a thickened soft tissue mass occupying space in the cervix (approximately 4.6*3.8*5.3 cm) (Figure 1A), which was consistent with changes associated with cervical cancer. The adjacent vaginal wall was invaded, and the uterine wall possibly was invaded. A nodule (approximately 3.1*2.5 cm) was present near the left pelvic wall (Figure 1C), which was considered to be a metastatic lymph node. There also were several small but slightly enlarged lymph nodes in the bilateral pelvic wall and inguinal region. Based on the MRI findings, a diagnosis of a medium, poorly differentiated cervical SCC, stage IIIC1r (FIGO 2018), was made.
From November 4 to December 30, 2019, external pelvic intensity-modulated radiation therapy was performed once a day at 200 cGy/dose from Monday to Friday for a total of 25 doses. Sequential internal and external three-dimensional conformal radiotherapy was performed at 500 cGy/dose for five doses. On October 11, November 24, and January 4, 2020, three rounds of TC regimen chemotherapy were given. The MRI was repeated at the end of treatment, and the lesion had regressed satisfactorily (Figure 1B and D). Subsequently, the patient was followed regularly in our outpatient department.
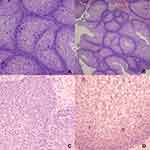
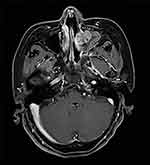
The patient’s serum SCCA remained significantly elevated and gradually increased during the outpatient review period from February 2020 to August 2020. Repeated gynecological examinations, cervical liquid-based cytology, chest computed tomography (CT), and abdominal MRI did not reveal any apparent abnormalities. A positron emission tomography-computed tomography (PET-CT) scan performed on September 15, 2020, revealed a soft tissue shadow in the left maxillary sinus that partially protruded into the left nasal cavity and was poorly delineated from the left superior and middle turbinate. The lesion was hypermetabolic (maximum and mean standardized uptake values [SUVmax and SUVmean] were 7.2 and 4.5, respectively), and a tumor lesion was not excluded (Figure 2). Fiberoptic nasopharyngoscopy revealed a rough protuberance of the local mucosa in the posterior portion of the middle nasal meatus on the left side, with an undefined nature. No other abnormalities were noted. A head MRI showed a soft tissue mass occupying the left maxillary sinus and the left nasal cavity (approximately 3.9*2.6 cm). The mass invaded the left middle and upper turbinates and the left ethmoid sinus and was poorly demarcated from the nasal septum (Figure 3). On October 12, 2020, the patient was subjected to a left sided medial maxillectomy through a trans-nasal endoscopic approach, by means of 0- and 70-degree rigid endoscopes. The left partial middle and inferior turbinate were excised. Intraoperative nasal endoscopy revealed that a greyish-white neoplasm was detected in the left middle nasal meatus and maxillary sinus with a papillary surface and no apparent necrotic pseudomembrane. The mass had invaded the uncinate process, but no other significant abnormalities were observed. Postoperative pathological examination of the neoplastic mass in the left maxillary sinus revealed a Schneiderian papilloma (Figure 4A and B). The SCCA level was assessed four weeks following the surgery and had decreased to a normal value (1.49 ng/mL).
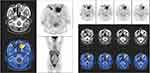 |
Figure 2 PET/CT image of the soft tissue shadow with increased SUV in the left maxillary sinus. |
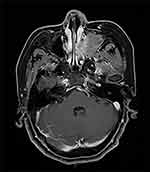 |
Figure 3 MRI image taken before the first sinus tumor resection. |
In the subsequent routine follow-up examination, the SCCA levels had gradually elevated again starting two months after the surgery and were consistently higher than normal (Figure 5). No abnormality was found in the cervix examinations. Therefore, an MRI of the sinuses was performed on May 27, 2021, that revealed a nodular soft tissue shadow (approximately 1.9*1.7*1.3 cm) in the left nasal cavity and the inner wall of the maxillary sinus in the previously resected area (Figure 6). Fiberoptic nasopharyngoscopy revealed an undefined mass in the left nasal cavity, and tumor recurrence was considered. Therefore, on June 18, 2021, tumor removal was performed under general anesthesia in our Head and Neck Surgery Department. After the lesion had been removed from the nasal cavity, the left inferior turbinate, left medial wall of the left maxillary sinus and inferior nasolacrimal duct were excised. Then the lesion in the maxillary sinus is radically removed using 70-degree rigid endoscopes. Intraoperative nasal endoscopy indicated that the nasal septum was slightly deviated. A greyish-white neoplasm was detected in the left middle nasal meatus and maxillary sinus with a papillary surface and no obvious necrotic pseudomembrane. The mass had invaded the middle part of the inferior nasal meatus, the inferior turbinate, and the nasolacrimal duct. No other significant abnormalities were observed. Postoperative pathological examination of the neoplasm in the left nasal cavity suggested it was a Schneiderian papilloma with squamous epithelium that exhibited papilloma-like hyperplasia (Figure 4C and D). Some areas exhibited an inverted growth pattern. The patient went to the Pathology Department of the West China Hospital of Sichuan University for pathological consultation. The diagnosis was inverted papilloma. Immunohistochemistry exhibited the following staining results: P16 (-), P53 (±), S-100 (-), Ki-67 (+), HPV (total) (-), and HPV6/11 (-). The patient’s SCCA level decreased to normal (1.09 ng/mL) two weeks following the second surgery. The patient has been in good condition, and the SCCA level has remained normal (Figure 5).
 |
Figure 6 MRI image taken before the second sinus tumor resection. |
Discussion and Conclusion
SCCA is genetically a member of the serine protease inhibitor (serpins) ovalbumin family and is transcribed by two highly homologous genes, SCCA1 and SCCA2.12 At the macromolecular level, SCCA is a subcomponent of tumor-associated antigens derived from SCC tissue and was first isolated from cervical SCC tissue.13 Elevated SCCA can be detected in SCCs of the tongue, esophagus, tonsil, epidermal follicle, lung, and uterus.14 In clinical practice, serum SCCA based on ELISA detection is currently used as a valuable marker for lymph node metastasis, treatment response, and tumor recurrence in SCCs of the cervix, lung, head and neck, esophagus, and liver. It has been shown that SCCA can also be detected in some other cells not tumoral cells such as peripheral T-lymphocytes, and one study found that serum SCCA1 levels were not associated with tissue-based expression.15 Currently, SCCA is recognized as a relatively specific disease-related indicator for cervical SCC and has substantial clinical significance for the diagnosis, efficient monitoring, and prognosis of the disease. SCCA levels also are an important indicator for patients with cervical SCC for treatment follow-up. The SCCA level of the patient in this report was significantly elevated at the time of her initial diagnosis and was more than ten times the upper limit of the normal value. During the chemoradiotherapy, the cervical SCC lesions regressed satisfactorily. However, the SCCA levels showed only a slight decline and remained elevated (14.62 ng/mL) at the end of treatment. During the subsequent months of outpatient follow-up, the SCCA level remained elevated (>10 ng/mL) and gradually increased. We first considered the possibility of cervical SCC progression, but no abnormalities were discovered in related examinations. We performed a whole-body PET/CT scan on the patient and found a metabolically active soft tissue shadow in the left maxillary sinus. After surgical resection, the patient’s SCCA level rapidly dropped to normal (1.49 ng/mL). Subsequently, SIP recurrence was determined due to a slow and gradual increase in SCCA levels. Following the second surgical excision, the SSCA levels rapidly decreased to normal. Thus, as a routine and relatively specific indicator of cervical SCC, SCCA appeared to be less impacted by the progression of cervical SCC, a malignant tumor, than the benign tumor SIP. This prompted us to consider the possible associations between SCCA expression and cervical SCC and SIP.
SP was first reported in 1854 by Ward and was thought to originate from Schneiderian epithelium located in the sinuses.16 The cause of the disease is unknown, but different etiologies have been postulated, including human papillomavirus (HPV) infection, chronic inflammation, environmental pollution, and occupational exposures (such as welding fumes, nickel compounds, and organic solvents).17 SP usually occurs in the lateral nasal wall, ethmoid sinus, and maxillary sinus and rarely in the frontal and sphenoid sinuses.18,19 The disease characteristics include a destructive growth pattern, high recurrence rate, and common malignant transformation. Currently, the preferred treatment is endoscopic sinus resection.20 However, even after extensive resection of the tumor, there is still a risk of recurrence or malignancy. Moreover, the recurrence rate (RR) of SIP is primarily affected by the location of the tumor growth and invasion.
In 2000, Krouse proposed a staging system based on the extent of tumor involvement observed on endoscopic examination of the nasal cavity and evaluation of examination images.21 One study indicated that in the Krouse staging system, as the invasion of specific areas changes from stage I to IV, the postoperative RR gradually increases.22 Thus when preoperative imaging reveals that the tumor has invaded the maxillary sinus, frontal sinus, or sphenoid sinus, the surgeon should pay particular attention to the thoroughness of the tumor resection and consider the possibility of postoperative recurrence. The surgical approach also affects the RR of SIP. This systematic review indicated that the endoscopic approach, which has a lower RR, is a favorable treatment option compared to external approaches.20 In addition, the recurrence rate of SIP is related to other factors such as tumor biological variation.23
Early diagnosis, treatment, and close postoperative follow-up are essential to improve the prognosis of patients. Therefore, it is critical to find disease-specific indicators to assess the efficacy and timely detection of tumor recurrence. So far, SCCA has not been used as a routine monitoring indicator of SIP. Several studies have reported that serum SCCA was elevated in patients with SIP and decreased after treatment.24–28 Yamashita et al studied 30 SIP patients undergoing surgical treatment from January 2006 to January 2015.28 Twenty-five patients exhibited elevated SCCA levels, with a median preoperative SCCA serum level of 2.4 ng/mL (interquartile range 1.7–5.2 ng/mL) and 1.0 ng/mL (interquartile range 0.8–1.4 ng/mL) in the postoperative period. There was a statistically significant difference in SCCA levels between the preoperative and postoperative phases of this study (P < 0.001). It was suggested that SCCA could be used as an indicator for diagnosing SIP and monitoring disease recurrence. However, another study reported that the preoperative SCCA levels were not associated with the risk of SIP recurrence, but the postoperative SCCA levels were positively correlated with the risk of disease recurrence (P < 0.001).29 Furthermore, patients with serum SCCA > 1.6ng/mL exhibited a high probability of tumor recurrence in the further. Therefore, it was recommended that the follow-up frequency should be increased, and more comprehensive examinations should be carried out for these patients.
Serum SCCA levels correlate with both cervical cancer and SIP and can vary with the occurrence and progression of the disease. However, for this patient, the entire course of the cervical cancer, which was a malignant tumor, appeared to have little impact on the changes in SCCA levels before and after treatment. On the other hand, SIP, a benign tumor, exhibited considerable influence on changes in SCCA levels. Therefore, based on the assessment of serum SCCA levels alone, it was challenging to determine whether the cervical SCC treatment was not effective or the serum SCCA levels remained elevated due to the presence of SIP. Serum SCCA levels reflect the total amount of serum SCCA1 and SCCA2, and there may be different mechanisms that result in elevated serum SCCA in benign tumors versus SCC. Yasumatsu et al reported that SCCA1 was more strongly expressed in SIP tissues than in SCC tissues.30 In contrast, SCCA2 was predominantly expressed in SCC tissues. The results of the Yasumatsu study are consistent with these previous studies of cervical cancer that found SCCA2 levels were more strongly expressed in malignant cervical tissues than in normal tissues.30,31
Appropriate tumor markers can help clinicians identify various neoplastic diseases, including SIP and cervical cancer. We could have determined whether SIP or cervical SCC played a major role in the increase in serum SCCA concentrations by detecting SCCA1 and SCCA2 levels. However, this patient did not undergo SCCA1 and SCCA2 detection because this project was not carried out in our hospital. Thus, this patient might have had elevated SCCA1 levels due to SIP, and the changes in SCCA levels were due to SIP rather than cervical SCC.
HPV is a circular, double-stranded DNA virus that primarily infects epithelial cells of the skin and mucosa. It is a common pathogen of sexually transmitted infections, and more than 200 subtypes exist.32 HPV is divided into high-risk types that exhibit carcinogenicity and low-risk types without carcinogenicity. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 are the identified types with high risk of carcinogenicity. Persistent infection with one of these high-risk HPVs commonly leads to a range of cancers and are especially closely associated with the occurrence of cervical cancer. 71% of cervical cancers are caused by HPV 16 and 18 infection.33
HPV infection is a controversial risk factor for SIP but is now considered to play an important role in SIP development. Gupta summarized 26 studies of HPV infection in SIP patients from April 2012 onwards.34 Samples from 1416 SIP patients were analyzed, and 330 cases were HPV positive (23.3%). The average HPV positive detection rate in SIP patients in 26 studies ranged from 0% to 62%. Of the 80 SIP patients reported by Pähler Vor der Holte,35 38 cases were HPV positive (38.8%), and the most common HPV genotypes were HPV6 (21/80, 26.3%) and HPV16 (18/80, 22.5%), followed by HPV11 (10/80, 12.5%), HPV58 (4/80, 5%), HPV42 and HPV83 (1/80, 1.3%). They also compared recurrent SP with non-recurrent SP, where the positive detection rates for HPV infection in recurrent and non-recurrent SP were 64.3% (18/28) and 41.9% (36/86), respectively. Thus, it can be inferred that HPV infection is a risk factor for SIP and plays a role in promoting the recurrence of SIP, but the detection rate varies greatly among different studies. Therefore, the association is not as clear as the correlation between cervical cancer and HPV.
HPV is transmitted in three ways, sexual transmission, vertical transmission, and extragenital contact. The patient in this case presented with cervical cancer combined with SIP, for which HPV16 was the common risk subtype. Therefore, extragenital contact appeared to be the more plausible route of transmission for the nasopharyngeal HPV infection in this case, but oral sex has also been reported as a risk factor for HPV transmission.36 We speculated that it is possible that the HPV that caused cervical SCC reached the sinus region by some route, causing a local infection that contributed to the development of SIP.
At present, the patient has been followed for more than one year after the second surgery. The patient is generally in good condition, the SCCA levels have remained in the normal range, and no abnormalities have been found in subsequent examinations. This patient will be followed for additional time to observe the clinical outcome.
This case study highlights several points. First, SCCA is a tumor marker closely associated with cervical SCC and an important indicator for monitoring changes in cervical SCC. When the changes in SCCA levels are inconsistent with the prognosis of cervical SCC, we should be vigilant about considering the possibility of other diseases existing at other sites in the body, especially tumors associated with HPV infection. Therefore, after completion of radiotherapy and chemotherapy for cervical cancer and the cervical lesions and pelvic lymph node lesions have resolved satisfactorily and the SCCA levels remain elevated, other sites should be promptly investigated for the cause. These actions might lead to earlier detection and treatment of SIP. Second, when cervical SCC and SIP exist simultaneously, it appears that SIP exerts a greater impact on SCCA expression than cervical SCC and that the SCCA levels are more closely related to SIP. Therefore, we recommend that SCCA could be used as a routine monitoring indicator for SIP. If available, assessment of SCCA1 and SCCA2 should be utilized for better discernment. Finally, for SIP that exhibits a high recurrence rate, anti-HPV treatment could be considered following surgery to reduce the risk of recurrence. This might help patients avoid a second surgery within months, reducing the physical and psychological impact of multiple surgeries. In this case, the patient experienced some change in the shape of her nose following the second surgery, which might have been avoided if anti-HPV treatment had been used.
However, some practical limitations remain. Although we believe that SCCA1 is mainly expressed in SIP and SCCA2 is highly expressed in cervical cancer, there are few hospitals that can detect SCCA1 and SCCA2 proteins in real clinical practice.
Data Sharing Statement
The original data presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics Approval and Informed Consent
Writing and publishing this case report was approved by Sichuan Cancer Hospital, The affiliated Cancer Hospital, School of Medicine, University of Electronic Science and Technology. The ethical committee approval number is SCCHEC-02-2022-121.
Consent for Publication
The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We thank the relatives of the patients for allowing us to share their medical history and clinical course. The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi:10.1016/S2214-109X(19)30482-6
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
3. Borcoman E, Le Tourneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther Adv Med Oncol. 2017;9:431–439. doi:10.1177/1758834017708742
4. Bhatla N, Berek JS, Cuello Fredes M, et al. Corrigendum to “Revised FIGO staging for carcinoma of the cervix uteri”. Int J Gynaecol Obstet. 2019;147:279–280.
5. Buchwald C, Franzmann MB, Tos M. Sinonasal papillomas: a report of 82 cases in Copenhagen Country, including a longitudinal epidemiological and clinical study. Laryngoscope. 1995;105:72–79. doi:10.1288/00005537-199501000-00016
6. Thompson LDR, Franchi A. New tumor entities in the 4th edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Virchows Arch. 2018;472:315–330.
7. Strojan P, Ferlito A, Lund VJ, et al. Sinonasal inverted papilloma associated with malignancy: the role of human papillomavirus infection and its implications for radiotherapy. Oral Oncol. 2012;48:216–218. doi:10.1016/j.oraloncology.2011.10.009
8. Lawson W, Ho BT, Shaari CM, Biller HF. Inverted papilloma: a report of 112 cases. Laryngoscope. 1995;105:282–288. doi:10.1288/00005537-199503000-00011
9. Sbrana MF, Borges RFR, Pinna FR, Neto DB, Voegels RL. Sinonasal inverted papilloma: rate of recurrence and malignant transformation in 44 operated patients. Braz J Otorhinolaryngol. 2019;87:80–84. doi:10.1016/j.bjorl.2019.07.003
10. Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–286. doi:10.1007/s12105-014-0527-7
11. Maroldi R, Farina D, Palvarini L, Lombardi D, Tomenzoli D, Nicolai P. Magnetic resonance imaging findings of inverted papilloma: differential diagnosis with malignant sinonasal tumors. Am J Rhinol Allergy. 2004;18:305–310. doi:10.1177/194589240401800508
12. Schneider SS, Schick C, Fish KE, et al. A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc Natl Acad Sci USA. 1995;92:3147–3151. doi:10.1073/pnas.92.8.3147
13. Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;1977:1621–1628. doi:10.1002/1097-0142(197710)40:4<1621::AID-CNCR2820400435>3.0.CO;2-I
14. Yang D, Wang J, Zhang L. Serum SCCA levels in patients suffering cancers or other diseases. Prog Mol Biol Transl Sci. 2019;162:165–175.
15. Derakhshan S, Poosti A, Razavi AE, et al. Evaluation of squamous cell carcinoma antigen 1 expression in oral squamous cell carcinoma (tumor cells and peritumoral T-lymphocytes) and verrucous carcinoma and comparison with normal oral mucosa. J Appl Oral Sci. 2021;29:e20210374. doi:10.1590/1678-7757-2021-0374
16. Ward N. A mirror of the practice of medicine and surgery in the hospitals of London: London hospital. Lancet. 1854;64:480–482. doi:10.1016/S0140-6736(02)54853-4
17. Deitmer T, Wiener C. Is there an occupational etiology of inverted papilloma of the nose and sinuses? Acta Otolaryngol. 1996;116:762–765. doi:10.3109/00016489609137921
18. Barnes L, Bedetti C. Oncocytic schneiderian papilloma: a reappraisal of cylindrical cell papilloma of the sinonasal tract. Hum Pathol. 1984;15:344–351. doi:10.1016/S0046-8177(84)80033-7
19. Anari S, Carrie S. Sinonasal inverted papilloma: narrative review. J Laryngol Otol. 2010;124:705–715. doi:10.1017/S0022215110000599
20. Goudakos JK, Blioskas S, Nikolaou A, Vlachtsis K, Karkos P, Markou KD. Endoscopic resection of sinonasal inverted papillomas: systematic review and meta-analysis. Am J Rhinol Allergy. 2018;32:167–174. doi:10.1177/1945892418765004
21. Krouse JH. Development of a staging system for inverted papilloma. Laryngoscope. 2000;110:965–968. doi:10.1097/00005537-200006000-00015
22. Cannady SB, Batra PS, Sautter NB, Roh HJ, Citardi MJ. New staging system for sinonasal inverted papilloma in the endoscopic era. Laryngoscope. 2007;117:1283–1287. doi:10.1097/MLG.0b013e31803330f1
23. Minni A, Gera R, Bulgheroni C, et al. Endoscopic resection of sinonasal inverted papilloma: a multivariate retrospective analysis of factors affecting recurrence and persistence. Ear Nose Throat J. 2021;100:542S–548S. doi:10.1177/0145561319890454
24. Yasumatsu R, Nakashima T, Kuratomi Y, et al. Serum squamous cell carcinoma antigen is a useful biologic marker in patients with inverted papillomas of the sinonasal tract. Cancer. 2002;94:152–158. doi:10.1002/cncr.10144
25. Yasumatsu R, Nakashima T, Masuda M, et al. Clinical value of serum squamous cell carcinoma antigen in the management of sinonasal inverted papilloma. Head Neck. 2005;27:44–48. doi:10.1002/hed.20115
26. Suzuki M, Deng Z, Hasegawa M, Uehara T, Kiyuna A, Maeda H. Squamous cell carcinoma antigen production in nasal inverted papilloma. Am J Rhinol Allergy. 2012;26:365–370. doi:10.2500/ajra.2012.26.3797
27. Matoušek P, Zeleník K, Safarčík K, Cábalová L, Komínek P. Squamous cell carcinoma antigen as a marker of sinonasal inverted papilloma. Eur Arch Otorhinolaryngol. 2014;271:535–538. doi:10.1007/s00405-013-2604-z
28. Yamashita Y, Uehara T, Hasegawa M, et al. Squamous cell carcinoma antigen as a diagnostic marker of nasal inverted papilloma. Am J Rhinol Allergy. 2016;30:122–127. doi:10.2500/ajra.2016.30.4287
29. van Zijl FVWJ, Monserez DA, Korevaar TIM, et al. Postoperative value of serum squamous cell carcinoma antigen as a predictor of recurrence in sinonasal inverted papilloma. Clin Otolaryngol. 2017;42:528–535. doi:10.1111/coa.12757
30. Yasumatsu R, Nakano T, Sato M, et al. Combination of serum squamous cell carcinoma antigens 1 and 2 as potential diagnostic marker for sinonasal squamous cell carcinoma and inverted papilloma. Head Neck. 2018;40:2583–2589. doi:10.1002/hed.25351
31. Hsu KF, Huang SC, Shiau AL, et al. Increased expression level of squamous cell carcinoma antigen 2 and 1 ratio is associated with poor prognosis in early-stage uterine cervical cancer. Int J Gynecol Cancer. 2007;17:174–181. doi:10.1111/j.1525-1438.2006.00663.x
32. Gheit T. Mucosal and cutaneous human papillomavirus infections and cancer biology. Front Oncol. 2019;9:355. doi:10.3389/fonc.2019.00355
33. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi:10.1002/ijc.30716
34. Gupta R, Rady PL, Sikora AG, Tyring SK. The role of human papillomavirus in the pathogenesis of sinonasal inverted papilloma: a narrative review. Rev Med Virol. 2021;31:e2178. doi:10.1002/rmv.2178
35. Pähler Vor der Holte A, Fangk I, Glombitza S, Wilkens L, Welkoborsky HJ. Prognostic factors and risk factors for development and recurrence of sinonasal papillomas: potential role of different HPV subtypes. Eur Arch Otorhinolaryngol. 2020;277:767–775. doi:10.1007/s00405-019-05747-4
36. Sánchez-Vargas LO, Díaz-Hernández C, Martinez-Martinez A. Detection of human papilloma virus (HPV) in oral mucosa of women with cervical lesions and their relation to oral sex practices. Infect Agent Cancer. 2010;5:25. doi:10.1186/1750-9378-5-25
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.