Back to Journals » Journal of Inflammation Research » Volume 15
A Rare Case of Acute Infectious Purpura Fulminans Caused by Klebsiella Pneumoniae and Human Herpesvirus Type 5
Authors Li XL, Luan CY, Fan YJ, Lin XY, Jiang D, Su MX, Wang G, Yang X
Received 14 April 2022
Accepted for publication 29 June 2022
Published 26 July 2022 Volume 2022:15 Pages 4251—4260
DOI https://doi.org/10.2147/JIR.S369986
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiao-Lan Li,1 Chun-Yan Luan,1 Ying-Jun Fan,2 Xiao-Ying Lin,1 Dong Jiang,1 Mei-Xian Su,3 Gang Wang,4 Xu Yang5
1Department of Dermatology, The Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, People’s Republic of China; 2Department of Rheumatology, The Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, People’s Republic of China; 3Department of Emergency Critical Care Medicine, The Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, People’s Republic of China; 4Department of Intensive Care Unit, The Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, People’s Republic of China; 5Laboratory Bacteria Room, The Second Affiliated Hospital of Kunming Medical University, Kunming, 650101, People’s Republic of China
Correspondence: Xiao-Lan Li, Department of Dermatology, The Second Affiliated Hospital of Kunming Medical University, No. 374 of Dian-Mian Avenue, Wu-Hua District, Kunming, 650101, People’s Republic of China, Tel +86 871 63402212, Fax +86 871 65334416, Email [email protected]
Background: Purpura fulminans (PF), a rare, life-threatening disorder, is a hematological emergency in which there is skin necrosis, disseminated intravascular coagulation (DIC), and protein C deficiency. In PF, the skin necrosis and DIC are secondary to protein C deficiency. This may progress rapidly to multiorgan failure caused by the thrombotic occlusion of small- and medium-sized blood vessels.
Case Report: This article presents the case of a 22-year-old male with fever as well as necrotic and purpuric skin lesions. The ultrasound and computed tomography scans revealed infections in the skin wounds as well as venous microthrombosis and thrombosis in multiple intracranial and pulmonary vessels. The laboratory tests showed signs of sepsis, thrombocytopenia, an abnormal decrease in protein C and antithrombin III, DIC, multiple organ and system failures, gastric varices, and gastrointestinal hemorrhage. The blood, sputum, and secretions under the skin lesions were cultured and were positive for Klebsiella pneumoniae. The results of the high-throughput genetic testing of the pathogenic microorganism DNA were consistent. In addition, human herpesvirus type 5 was detected. The histopathological examination of the skin lesions revealed pathological features consistent with PF. After successful treatment by the departments of Dermatology, Emergency Critical Care Medicine, and the Intensive Care Unit, the patient was discharged after 67 days of hospitalization.
Conclusion: Adults with acquired protein C and/or S deficiency states, including certain bacterial and viral infections, who drink alcohol and take varieties of non-steroidal anti-inflammatory analgesics at the same time, may develop acute infectious PF. Clinicians should be aware of this for early diagnosis and treatment.
Keywords: acute infectious purpura fulminans, Klebsiella pneumoniae, human herpesvirus type 5, disseminated intravascular coagulation, multiple organ and system failures
Introduction
Purpura fulminans (PF) is a rare syndrome of intravascular thrombosis and skin hemorrhagic infarction and necrosis that usually occurs in children. It progresses rapidly, with vascular collapse and systemic disseminated intravascular coagulation (DIC). The pathogenesis of the disease has recently been classified according to the following three types of trigger mechanisms. (1) Neonatal PF is related to the genetic deficiency of natural anticoagulant proteins C and S; (2) idiopathic or chronic PF occurs after the incubation period of various viral infections; and (3) the most classic and common form of this disease, called acute infectious PF (AIPF), occurs when it is superimposed on bacterial infections; this kind of PF is related mainly to acquired protein C deficiency. Meningococcus and varicella virus are the two most common bacteria and trigger viruses. In addition, they may also be associated with Gram-negative bacilli, staphylococcus, streptococcus, rickettsia, and measles.1,2
Case Presentation
A 22-year-old male cook was admitted to the Dermatology Department of the Second Affiliated Hospital of Kunming Medical University with a 15-day history of fever as well as escharotic and purpuric skin lesions on his lower limbs. The patient had self-administered ibuprofen (sustained-release capsules) and diclofenac sodium enteric-coated tablets for one week, one tablet per day, to relieve fever and pain; in addition, the local hospital gave him cephalosporin antibiotics intravenously for four days. The administered medications did not seem to work. The patient had a history of alcoholism, drinking 50–600 mL of beer or hard liquor (with an alcohol level of 58) per day for two years.
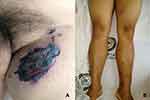
On admission, the patient’s body temperature (T) was 39°C, his pulse was 120 bpm, and his blood pressure was 154/108 mmHg. His height was 166 cm, and his weight was 90 kg, suggesting severe obesity according to Chinese weight standards. The physical examination found a black necrotic eschar with an area of approximately 3×7 cm in the center of the redness and swelling in his left groin area, at the root of the thigh. The texture was hard and difficult to peel, and there were subcutaneous nodules on the periphery (Figures 1A and B). The skin T of the lesion area was higher than the surrounding normal tissue. Several enlarged lymph nodes could be palpated in the groin on both sides. The lower limbs were scattered with purpuric and necrotic crusted lesions (Figure 1B). A color Doppler ultrasound examination revealed sonographic images of thrombosis in the small saphenous vein of the right lower limb, the calf segment of the bilateral great saphenous vein, the forearm segment of the cephalic vein of both arms, and the upper arm segment of the right cephalic vein. A computed tomography (CT) scan showed a large, fresh cerebral infarction, intracranial infection, lung infection, pleurisy, and pericardial effusion. A magnetic resonance imaging head scan with diffusion and lumbar spine analysis suggested large fresh infarctions in the left parietal frontal lobe and the occipital lobe.
The blood cell analysis showed the following: white blood cell (WBC) count 14.67 × 109/L; neutrophils (NEUT) 7.08 × 109/L; lymphocytes (LYMPH) 2.39 × 109/L; monocytes (MONO) 0.64 × 109/L; eosinophils (EO) 4.53 × 109/L; basophils 0.03 × 109/L; red blood cell (RBC) count 4.69 × 1012/L; hemoglobin (HGB) 148 g/L; and platelet (PLT) count 19 × 109/L. Coagulation showed a prothrombin time (PT) of 17.8 s, a PT ratio of 1.37, an international normalized ratio of 1.49, an activated partial thromboplastin time (APTT) of 50.9 s, a fibrinogen (FIB) of 2.00 g/L, a thrombin time of 17.0 s, a D-dimer of 44.28 ug/mL, an antithrombin III (AT) of 87.0%, and fibrin degradation products of >150.0 μg/mL.
On day two, the patient experienced pain in the skin lesions and in the back, headache with vertigo, sputum-free cough, diarrhea, and stomach ache, all gradually increasing in severity.
On day seven, the patient developed abdominal pain. Hematemesis was 20 mL, and blood in the stool was 1500 mL. Occult blood tests of fecal and stomach contents were both positive. An ordinary gastroscopy and duodenal examination showed gastrointestinal bleeding, but no bleeding points were found. A pulmonary artery CT angiography (CTA) showed multiple pulmonary embolisms in each lobe, segment, and sub-segment of both lungs. An abdominal CT revealed the following: (1) there was suspected liver damage; (2) the middle and right veins of the liver, the main portal vein, the left and right branches, the splenic vein, and the superior mesenteric vein had multiple embolisms; (3) the left inguinal area had obvious swelling, and the density of the subcutaneous fat was mixed. This was considered inflammatory.
On day 10, the patient had difficulty breathing, irritability, and a progressive decline in blood oxygen saturation and partial pressure of oxygen. Respiratory failure was considered. He was transferred to the Department of Emergency Critical Care Medicine and was injected with sedative drugs. A right subclavian vein puncture and a catheterization were performed to give intravenous hypernutrition. There were no special findings in the fiberoptic bronchoscopy. A blood biochemical analysis showed liver function damage, renal function damage, hypokalemia, hyperchloremia, and hypernatremia; the blood gas indicated a decompensated period of metabolic acidosis. The patient was in a continuous coma without sedation medicine with a T of 37.6°C–39.5°C.
On day 20, the patient was given continuous renal replacement therapy. The patient’s consciousness improved, and he was able to respond. He had a body T of 37.6°C–38.5°C. He defecated tar-like stools with positive occult blood test twice for a total of 800 mL. The PLT increased to 61 × 109/L, after which the patient was extubated, and oxygen was administered by mask.
On day 22, the patient was returned to the Dermatology Department.
On day 24, his blood chloride increased, his oxygen partial pressure was 48%, his carbon dioxide partial pressure was 23%, his HGB was 48 g/L, and he was transferred to the Intensive Care Unit (ICU). In addition, femoral artery puncture catheterization and monitoring by Pulse index Continuous Cardiac Output (PiCCO) (Pulsion Medical Systems, Germany), radial artery puncture catheterization and invasive blood pressure monitoring, and peripheral puncture central venous catheterization were given.
On day 30, the patient still had active bloody stools (170 mL) and a bruise (15 × 20 cm) with edema appeared on the right upper limb.
On day 36, his T was 37°C–39°C, and his HGB was 80 g/L. He was returned to the Dermatology Department.
On day 41, red liquid drained from the patient’s gastric tube, accompanied by palpitations, shortness of breath, and convulsions after a little activity.
On day 44, his breathing difficulty symptoms worsened. The mask was given at 66–78% oxygen saturation, and the patient was transferred to the ICU again. He was given thoracentesis catheter drainage, tracheal intubation, and mechanical ventilation.
On day 52, his condition gradually improved. His T was 37°C–37.8°C, and the PLT was 355 × 109/L. His coagulation function was improved.
On day 56, the patient was in a stable condition with normal body T, and he was returned to dermatology.
On day 57, a gastroscopy showed varicose veins in the fundus of the stomach and chronic non-atrophic gastritis.
On day 66, a portal venous system CTA showed the following: (1) the manifestations of splenic infarction and the (2) thrombosis of the portal vein, hepatic arteriovenous, and inferior vena cava had disappeared.
On day 67, the patient’s thigh root skin lesion area was significantly reduced, the texture became soft, and there was no tenderness. The purpura-like skin lesions on both calves had completely subsided, his vital signs were stable, and all laboratory indicators were stable Additionally, his weight had dropped to 46 kg. Therefore, the patient was discharged.
Daily laboratory tests showed the following: continually increasing WBC and EO counts; thrombocytopenia (Figure 2A); elevated PT/APTT; elevated thrombolytic dimer; reduced AT; low FIB, a concern for DIC (Figure 2B); decreased serum total protein and albumin; abnormally elevated transaminase; high levels of serum bilirubin and bile acids, suggesting liver damage (Figure 2C); abnormally elevated muscle enzymology (Figure 2D); abnormally elevated creatinine and uric acid causing a concern for renal function failure (Figure 2D); abnormally elevated fasting blood glucose and serum iron (Figure 2E); proteinuria; fecal occult blood; and an abnormal decrease in protein C of 43.1% (70.0–140.0).
All the autoantibody profiles were negative. Complement C3 reduced to 0.43 g/L (0.79–1.52 g/L), and high-sensitivity C-reactive protein increased between 97.10–354.00 mg/L (<10 mg/L).
Pathogen culture: The blood, sputum, and secretions under the skin lesions were cultured for a variety of bacteria, including multi-drug-resistant bacteria (Table 1). Three specimens were positive for Klebsiella pneumoniae (Figure 3A). The results of the high-throughput genetic testing of the pathogenic microorganism DNA were consistent. Human herpesvirus type 5 was detected in both the patient’s blood and skin lesions (Table 2). Additionally, an oral mucosa fungus microscopic examination was positive (Figure 3B).
 |
Table 1 Pathogen Culture and DNA Quantification |
 |
Table 2 High-Throughput Genetic Testing of Pathogenic Microorganism DNA |
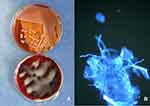 |
Figure 3 (A) Klebsiellasubsp. from secretions under the scab of the lesion on a MacConkey agar plate (top) and on a blood plate (bottom). (B) Positive oral mucosa fungus microscopic examination. |
Histopathological examination of the skin lesions revealed the following: (1) the formation of epidermal serous callus; (2) extensive liquefaction and degeneration of the basal layer; (3) stenosis and occlusion of most of the vascular lumen of the dermis and subcutaneous tissue; (4) fibrinoid necrosis of the blood vessel walls; (5) inflammatory thrombosis filling in part of the blood vessel cavities; (6) extensive dermis collagen sheet necrosis; (7) hyperplasia of the subcutaneous superficial adipose tissue; (8) necrosis of the fat cells in the lobules; and (9) mixed inflammatory cell infiltration (Figures 4 and 5).
Rescue and treatment: (1) Continuous renal replacement therapy, tracheal intubation, mechanical ventilation, continuous alternating infusion of ordinary frozen plasma and cryoprecipitate clotting factors, and suspended RBC and PLT apheresis were administered when necessary. (2) A variety of sensitive and broad-spectrum antibiotics were utilized and administered based on experience or drug sensitivity results. (3) Medium-dose glucocorticoids (ie, methylprednisolone), intravenous immunoglobulin, hemostasis, acid suppression and stomach protection, liver protection, etc., were given (Figure 6: Main medications and treatments). After treatment, the patient’s condition improved, and all abnormal laboratory indicators mentioned above gradually returned to their normal ranges (Figure 2: Changes in laboratory examination results).
The main diagnoses were AIPF, acute cellulitis, sepsis, diffuse intravascular coagulation, multiple organ failure, acute upper gastrointestinal bleeding, acute respiratory distress syndrome, pulmonary embolism, extensive cerebral infarction, hepatic vein thrombosis, portal vein thrombosis, splenic vein thrombosis, superior mesenteric vein thrombosis, deep vein thrombosis of upper limbs and lower extremities formation, lung infection, oral Candida infection, and liver damage/abnormal liver function.
Discussion
When a patient is diagnosed with AIPF, their skin lesions appear as punctuated purpura that quickly turn into large, fused ecchymosis areas. With further necrosis, a typical hard eschar forms, which is characteristic of the disease. Bilateral symmetric gangrene is also a feature of PF, which usually requires amputation. In fact, some studies indicate that the amputation rate is 19%.3 Sepsis, DIC, and concomitant multisystem organ failure are the biggest obstacles to survival, with a reported mortality rate as high as 50%.4
This article presents a case of AIPF due to Klebsiella pneumoniae, which is rarely associated with PF. Indeed, Gram-negative rods are less commonly involved. Klebsiella quasipneumoniae is a kind of non-motile, non-spore-forming Gram-negative orthobacteria, isolated from human infection samples, that includes two subspecies: Klebsiella quasipneumoniae subsp. quasipneumoniae subsp. nov and Klebsiella quasipneumoniae subsp. quasipneumoniae subsp. It is a typical conditional pathogen, and is often already present in the host when the host’s resistance drops, causing an outbreak. Klebsiella pneumoniae is a common cause of infectious disease in hospitals and community settings and is associated with a variety of clinical conditions, including bacterial pneumonia, meningitis, endocarditis, liver abscess, intra-abdominal infection, urinary tract infection, and bloodstream infection. Klebsiella pneumoniae is reported to be the second most common cause of Gram-negative bacteremia. Recently, various microbial factors and virulence genes of Klebsiella pneumoniae have been reported to be associated with its specific clinical features and a high mortality rate.5,6
Tsubouchi et al reported that a 75-year-old woman died of skin hemorrhage, purpura, gangrene, and sepsis caused by Klebsiella pneumoniae infection.7 Iacovelli et al reported that ceftazidime/avibactam was used to successfully treat a carbapenem enzyme-producing case of pneumonia, septic thrombophlebitis, and right ventricular wall endocarditis caused by Klebsiella.8 Nguyen et al found that a patient with acute liver failure, possibly due to acetaminophen overdose and a Klebsiella pneumoniae bacterial infection, was found to have rapidly progressive purpura. This patient suffered from acral gangrene and DIC, with decreased protein C and S activity. A skin biopsy revealed microthrombi in the dermal blood vessels.9 Olowu reported Klebsiella-induced PF in a 3.5-year-old Nigerian girl with fever, vomiting, diarrhea, difficulty with breathing, swollen feet, and gangrenous toes. Amputations of the fourth and fifth toes of her left foot occurred on day 18 of the illness.10 Disse et al reported that a 17-day-old neonate suffering sepsis-associated PF due to Klebsiella oxytoca was given broad-spectrum antibiotics, ventilation, diuretics, protein C substitution, and burn protocol. With this treatment, the patient’s limbs were successfully preserved with scarring.11
Similar to the above cases, this patient, who was simultaneously infected with Klebsiella pneumoniae in the blood, skin wounds, and lungs, rapidly progressed with small vessel microthrombosis, multiple large vein thrombosis, pulmonary embolism, and extensive cerebral infarction. He also presented with a very severe form of DIC, which involves disturbances in the balance of procoagulant and anticoagulant endothelial cell activities,12 leading to prolonged PT and PTT. However, the thrombocytopenia is common to all cases. This disorder is triggered by bacterial endotoxins, which mediate vascular injury through a variety of effects on the alternate pathway of complement, NEUT, endothelial cells, factor XII, and MONO. Activated MONO secrete proinflammatory cytokines, such as TNF-ɑ, IL-1β, IL-6, IL-12, and INF-γ, which consume proteins C, S, and AT. They also play an important role in the mediation of the biological effects of bacterial endotoxins. These factors subsequently release other cellular mediators and activate NEUT and endothelial cells; these events invariably lead to the adhesion of the activated NEUT to the endothelial cells, thereby causing vascular injury and DIC with varying degrees of thrombosis and/or bleeding. In AIPF, the degree of thrombosis is usually profound and includes serious ischemic injuries to tissues and organs.13
Human beta-herpesvirus 5, also known as human cytomegalovirus (HCMV), is a common infection in the human body. Herpesviruses persist in the host due to the switch between two modes: recessive infection and cytolytic infection.14 The principal modes of transmission of HCMV are through the respiratory tract or close contact, and its main target cells are vascular endothelial cells, which act as a viral reservoir. Multiple factors can cause persistent HCMV infection, resulting in endothelial cell dysfunction and activation of proinflammatory signaling systems, including the NF-κB, SP-1, PI3K, and PDGF receptors. This infection increases the molecular expression of endothelial cell adhesion; it also alters the ability of MONO and macrophages to lyse proteins, recruit new MONO from the bloodstream, and contact already infected endothelial cells of HCMV transfer virus particles to MONO and diffuse powder. Conversely, endothelial cell injury promotes thrombosis, which becomes a predisposing factor for cardiovascular and cerebrovascular diseases.15
The experiment by Rahbar et al found that after HCMV infected vascular endothelial cells in vitro, PLT adhesion and aggregation were significantly higher than those of the uninfected cells, and the von Willebrand factor, vascular cell adhesion molecule 1, sialyl Lewis X, and intercellular adhesion molecule 1, etc., were markedly elevated, demonstrating increased expression on endothelial cells. Both may increase thrombosis and leukocyte adhesion. Increased thrombosis is dependent on active viral replication and can be inhibited by foscarnet and ganciclovir.16
Rinaldi et al reported venous thromboembolism in a 62-year-old woman and a 20-year-old Caucasian woman due to acute cytomegalovirus infection, possibly due to HCMV-induced endothelial damage and coagulopathy.14
It has also been observed that long-term drinkers taking therapeutic acetaminophen may result in an overdose, leading to severe hepatotoxicity. Patients with resulting liver damage may develop secondary hepatic dysfunction and coagulopathy with impaired protein synthesis, leading to acquired protein C and S deficiency, or they may be potential candidates for this deficiency.9,17 Jack et al reported a 32-year-old woman was instructed to take two extra-strength acetaminophen tablets every four to six hours for minor aches and pains after a car accident. At the same time, this patient increased her usual alcohol consumption over the ensuing three days. Eight hours before her admission to the hospital, the patient noted rapidly spreading purpuric lesions on her hands and feet with tight edema. Laboratory tests revealed elevated liver enzyme values and reduced protein C.17
For other non-steroidal anti-inflammatory drugs, Kosaraju et al described a case of a healthy woman who developed DIC after intramuscular administration of a single 60-mg intramuscular dose of ketorolac. She had large areas of ecchymosis all over her face, trunk, and extremities.18 The patient in the present study was taking normal doses of ibuprofen and diclofenac sodium for one week at the beginning of the disease, but still did not stop drinking alcohol, so this event may be one of the triggers for the complex pathogenesis. A review of the relevant literature showed only one other case description of diclofenac-related PF with DIC.19
Conclusion
The clinical manifestations of AIPF are skin purpura, DIC, and microvascular thrombosis in the skin and other tissues. In addition, even larger organ thrombosis and hemorrhagic infarction may occur. Thrombosis, one of the consequences of AIPF, leads to multiple organ failure and a high mortality rate. A delayed diagnosis of AIPF can lead to severe adverse clinical consequences, such as amputation and death. Therefore, a special emphasis on testing for AIPF should be placed on adults with acquired protein C and S deficiency states, including certain bacterial and viral infections, that also drink alcohol and simultaneously take different varieties of non-steroidal anti-inflammatory analgesics, as these factors may lead to AIPF. Most importantly, the timely and repeated administration of intravenous human immunoglobulin, cryoprecipitate coagulation factors, and apheresis PLTs can improve the prognosis and reduce the disease’s mortality. It is hoped that this complex case can inspire multidisciplinary clinical thinking by physicians and dermatologists.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from the patient.
Consent for Publication
The patient signed a document of informed consent.
Acknowledgments
Histopathology Department, Clinical Laboratory of the Second Affiliated Hospital of Kunming Medical University.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82160596).
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96(11):1066–1071. PMID: 21233082. doi:10.1136/adc.2010.199919
2. Andreasen TJ, Green SD, Childers BJ. Massive infectious soft-tissue injury: diagnosis and management of necrotizing fasciitis and purpura fulminans. Plast Reconstr Surg. 2001;107(4):1025–1035. PMID: 11252099. doi:10.1097/00006534-200104010-00019
3. Besner GE, Klamar JE. Integra artificial skin as a useful adjunct in the treatment of purpura fulminans. J Burn Care Rehabil. 1998;19(4):324–329. PMID: 9710731. doi:10.1097/00004630-199807000-00010
4. Sheridan RL, Briggs SE, Remensnyder JP, Tompkins RG. Management strategy in purpura fulminans with multiple organ failure in children. Burns. 1996;22(1):53–56. PMID: 8719318. doi:10.1016/0305-4179(95)00078-x
5. Imai K, Ishibashi N, Kodana M, et al. Clinical characteristics in blood stream infections caused by Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae: a comparative study, Japan, 2014–2017. BMC Infect Dis. 2019;19(1):946. PMID: 31703559; PMCID: PMC6842162. doi:10.1186/s12879-019-4498-x
6. Breurec S, Melot B, Hoen B, et al. Liver abscess caused by infection with community-acquired Klebsiella quasipneumoniae subsp. quasipneumoniae. Emerg Infect Dis. 2016;22(3):529–531. PMID: 26890371; PMCID: PMC4766917. doi:10.3201/eid2203.151466
7. Tsubouchi N, Tsurukiri J, Numata J, Sano H. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58(12):1801–1802. PMID: 30713331; PMCID: PMC6630121. doi:10.2169/internalmedicine.2350-18
8. Iacovelli A, Spaziante M, Al Moghazi S, Giordano A, Ceccarelli G, Venditti M. A challenging case of carbapenemase-producing Klebsiella pneumoniae septic thrombophlebitis and right mural endocarditis successfully treated with ceftazidime/avibactam. Infection. 2018;46(5):721–724. PMID: 29926399. doi:10.1007/s15010-018-1166-9
9. Nguyen V, Myint JA, Philipneri M. Purpura fulminans in the setting of Klebsiella Pneumoniae bacteremia and acetaminophen overdose. Cureus. 2020;12(11):e11633. PMID: 33376646; PMCID: PMC7755612. doi:10.7759/cureus.11633
10. Olowu WA. Klebsiella-induced purpura fulminans in a Nigerian child: case report and a review of literature. West Afr J Med. 2002;21(3):252–255. PMID: 12744583. doi:10.4314/wajm.v21i3.28043
11. Disse SC, Meyer S, Baghai-Arassi A. Sepsis-associated purpura fulminans due to Klebsiella Oxytoca. Dtsch Arztebl Int. 2018;115(46):784. PMID: 30602411; PMCID: PMC6329368. doi:10.3238/arztebl.2018.0784a
12. Darmstadt GL. Acute infectious purpura fulminans: pathogenesis and medical management. Pediatr Dermatol. 1998;15(3):169–183. PMID: 9655311. doi:10.1046/j.1525-1470.1998.1998015169.x
13. Edlukudige Keshava V, Bhavsar HV, Ghionni N, Baba RH, Mcnamee W. Overwhelming sepsis due to capnocytophaga canimorsus in an immunocompetent individual: a rare case study. Cureus. 2020;12(9):e10177. PMID: 33029457; PMCID: PMC7529488. doi:10.7759/cureus.10177
14. Rinaldi F, Lissandrin R, Mojoli F, et al. Acute cytomegalovirus infection as a cause of venous thromboembolism. Mediterr J Hematol Infect Dis. 2014;6:e2014041. PMID: 24959338; PMCID: PMC4063613. doi:10.4084/MJHID.2014.041
15. Nikitskaya E, Lebedeva A, Ivanova O, et al. Cytomegalovirus productive infection is associated with acute coronary syndrome. J Am Heart Assoc. 2016;5(8):e003759. doi:10.1161/JAHA.116.003759
16. Rahbar A, Söderberg-Nauclér C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79(4):2211–2220. PMID: 15681423; PMCID: PMC546536.17. doi:10.1128/JVI.79.4.2211-2220.2005
17. Guccione JL, Zemtsov A, Cobos E, Neldner KH. Acquired purpura fulminans induced by alcohol and Acetaminophen. Successful treatment with heparin and vitamin K. Arch Dermatol. 1993;129(10):1267–1269. PMID: 8215490. doi:10.1001/archderm.1993.01680310037005
18. Kosaraju N, Korrapati V, Thomas A, James BR. Adult purpura fulminans associated with non-steroidal anti-inflammatory drug use. J Postgrad Med. 2011;57(2):145–146. PMID: 21654144. doi:10.4103/0022-3859.81876
19. Hengge UR, Jochum C, Tschakarjan E, et al. Purpura fulminans fatale folge einer alltäglichen therapie? [Purpura fulminans. A fatal consequence of a widely used medication?]. Hautarzt. 2002. 53(7):483–487. German. PMID: 12219272. doi:10.1007/s00105-002-0346-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.