Back to Journals » Medical Devices: Evidence and Research » Volume 11
A randomized, controlled trial of Veriset™ hemostatic patch in halting cardiovascular bleeding
Authors Glineur D, Hendrikx M, Krievins D , Stradins P, Voss B, Waldow T, Haenen L, Oberhoffer M, Ritchie CM
Received 6 July 2017
Accepted for publication 18 November 2017
Published 8 March 2018 Volume 2018:11 Pages 65—75
DOI https://doi.org/10.2147/MDER.S145651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
David Glineur,1 Marc Hendrikx,2 Dainis Krievins,3 Peteris Stradins,3 Bernhard Voss,4 Thomas Waldow,5 Luc Haenen,6 Martin Oberhoffer,7 Caroline M Ritchie8
1Saint Luc Cliniques Universitaires, Brussels, Belgium; 2Faculty of Medicine and Life Sciences, Jessa Hospital, Hasselt University, Hasselt, Belgium; 3Pauls Stradins Clinical University Hospital, Riga, Latvia; 4German Heart Center Munich, Department of Cardiovascular Surgery, Technische Universität München, Munich, Germany; 5Heart Center Dresden GmbH, University Hospital Dresden, Dresden, Germany; 6Imelda Hospital, Bonheiden, Belgium; 7Asklepios Klinik St. Georg, Herzchirurgische Abteilung, Hamburg, Germany; 8Covidien, Medical Affairs, Bedford, MA, USA
Background: Obtaining hemostasis during cardiovascular procedures can be a challenge, particularly around areas with a complex geometry or that are difficult to access. While several topical hemostats are currently on the market, most have caveats that limit their use in certain clinical scenarios such as pulsatile arterial bleeding. The aim of this study was to assess the effectiveness and safety of Veriset™ hemostatic patch in treating cardiovascular bleeding.
Methods: Patients (N=90) scheduled for cardiac or vascular surgery at 12 European institutions were randomized 1:1 to treatment with either Veriset™ hemostatic patch (investigational device) or TachoSil® (control). After application of the hemostat, according to manufacturer instructions for use, time to hemostasis was monitored. Follow-up occurred up to 90 days post-surgery.
Results: Median time to hemostasis was 1.5 min with Veriset™ hemostatic patch, compared to 3.0 min with TachoSil® (p<0.0001). Serious adverse events within 30 days post-surgery were experienced by 12/44 (27.3%) patients treated with Veriset™ hemostatic patch and 10/45 (22.2%) in the TachoSil® group (p=0.6295). None of these adverse events were device-related, and no reoperations for bleeding were required within 5 days post-surgery in either treatment group.
Conclusion: This study reinforces the difference in minimum recommended application time between Veriset™ hemostatic patch and TachoSil® (30 s versus 3 min respectively). When compared directly at 3 min, Veriset™ displayed no significant difference, showing similar hemostasis and safety profiles on the cardiovascular bleeding sites included in this study.
Keywords: surgical bleeding, cardiac surgery, aortic valve replacement, CABG
Background
Uncontrolled bleeding is a major challenge for surgeons, with both patient safety and economic implications.1,2 This is especially true in the cardiovascular field, due to anticoagulation and anti-aggregation treatment taken prior to and during surgery and the systolic pulsatility present in the arterial system, which increases stress on sutures. Cardiopulmonary bypass (CPB) is often used during these procedures, affecting the intrinsic coagulation pathway rendering the control of bleeding more challenging.3 In fact, studies have shown that these interventions are associated with increased blood loss and post-operative mortality.4,5 Thus, the control of bleeding in these situations requires both an urgent and conservative approach. While there are many topical hemostats that have shown efficacy when applied to cardiovascular tissue,6 their caveats (e.g., need of a dry field, efficacy only on low pressure bleeding) support the need to develop a topical hemostat that is easy to use, safe, and effective in the cardiovascular patient population.
Veriset™ (Covidien; Mansfield, MA, USA) hemostatic patch promotes hemostasis through a dual mode of action, serving as a tamponade to physically stem blood flow, while concentrating platelets and other clotting factors at the bleeding site to accelerate coagulation. Veriset™ hemostatic patch is left in place after hemostasis is obtained, and is completely absorbed within 4 weeks. In two previous clinical trials, one in hepatic tissue and the other in soft tissue, no adverse events (AEs) related to Veriset™ hemostatic patch were reported, and quick control of bleeding was obtained.7,8 In order to obtain clinical results on the device when applied to cardiovascular tissue, we performed a randomized, controlled, patient-blinded study comparing the safety and efficacy of Veriset™ hemostatic patch to that of TachoSil® (Nycomed; Zürich, Switzerland) (currently approved for cardiovascular hemostasis), with a follow-up of 90 days post-surgery.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and all local regulatory requirements. Copies of the protocol, proposed informed consent forms, other written subject information, and other study-related documents, as required by the ethics committees, were submitted to the Independent Ethics Committees (IECs) for approval. IEC approval was obtained before enrollment of subjects into the study and shipment of the study device. A list of participating IECs and their associated clinical trial sites is provided in Table S3.
Methods
Study design
This was a premarket, prospective, randomized, single-blind study to compare Veriset™ hemostatic patch to TachoSil® as an adjunct to hemostasis in patients undergoing cardiovascular surgery (clinical trial registration number: NCT01639833). Patients at 12 European institutions scheduled for open cardiac or vascular surgery involving the aorta, surface of the heart, or coronary artery bypass grafting (CABG), and who provided informed consent, were considered for enrollment. During surgery, treatment at the target bleeding site (TBS) was randomized 1:1 using a sealed envelope process to Veriset™ hemostatic patch or TachoSil®, and the time required to obtain hemostasis was monitored. Safety was assessed up to 90 days post-surgery. The clinical study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. All European and national regulations were adhered to. The datasets generated and/or analyzed during the current study are available in the Clinical Trial repository: https://clinicaltrials.gov/ct2/show/NCT01639833?term=NCT01639833&rank=1.
Study participants
Patients who met certain inclusion criteria, but none of the exclusion criteria, were enrolled in the study (Table 1). An appropriate TBS was defined as an area of bleeding on the aorta, coronary vessels, or surface of the heart, where hemostasis by conventional methods was deemed ineffective, impractical, or potentially detrimental, of Type 2 (oozing/mild) or Type 3 (moderate) bleeding severity,9 and where it was possible to hold pressure on Veriset™ hemostatic patch or TachoSil® for at least 3 min. The bleeding severity levels are based on a published scale in order to increase objectivity; bleeding levels were assigned prior to randomization in order to minimize investigator bias with a Type 1 (no visible bleeding) and Type 4 (uncontrolled bleeding requiring immediate conventional surgical intervention) excluded from the study. Ninety patients were enrolled beginning on August 2, 2012, with the final follow-up visit on December 16, 2013.
  | Table 1 Criteria for study enrollment Abbreviations: CABG, coronary artery bypass grafting; TBS, target bleeding site. |
Materials
Veriset™ hemostatic patch (Covidien), a device composed of polyethylene glycol (PEG) and oxidized cellulose, and TachoSil® (Nycomed), a patch composed of equine collagen coated with human fibrinogen and thrombin, were provided by the study sponsor. There are no known contraindications for Veriset™ hemostatic patch. TachoSil® is contraindicated for intravascular application to avoid life-threatening thromboembolic events, and in patients with known hypersensitivity to human blood products or horse proteins. Both hemostats were to be used in accordance with the manufacturers’ instructions for use and could be cut to size. All other supplies used in the surgical procedure or for assessment were those standardly used by the institution at which the surgery was performed.
Surgical procedure
Patients underwent open cardiac or vascular surgery according to the appropriate standard procedures and practices of the institution at which the surgery was performed. Protamine reversal was to occur prior to randomization. Once an appropriate TBS was identified, the patient was randomized to receive treatment with either Veriset™ hemostatic patch or TachoSil®. After application of Veriset™ hemostatic patch, direct pressure was applied. Hemostasis was assessed every 30 s after application until the 3 min time point, and then at 1 min intervals until hemostasis was achieved, up to 10 min. After application of TachoSil®, pressure was maintained on the device and hemostasis was not assessed until 3 min after application, per the manufacturer’s instructions for use. If hemostasis was not obtained at 3 min, continued pressure was applied to the device, and hemostasis was assessed at 1 min intervals up to 10 min. If hemostasis was not obtained within 5 min of application of either device, other hemostatic measures could be used as rescue therapy at the discretion of the investigator. Comparable pre-clinical application images can be found in Howk et al. 10 During CABG procedures, the location of patch application was at the discretion of the surgeon and not specifically directed by the study protocol, and the force applied to stop suture hole bleeding during the 30 s intervals was not detrimental for graft patency.
Outcome measures
The primary safety endpoint was the proportion of patients with serious adverse events (SAEs) up to 30 days post-surgery, and the secondary safety endpoint was the proportion of patients requiring a reoperation for bleeding complications up to 5 days post-surgery. Although patients were monitored for 90 days, the Veriset™ patch is absorbed within 30 days making it an appropriate safety endpoint for the device. The safety endpoints were evaluated based on non-inferiority to the control and were tested from an intention to treat perspective. The primary effectiveness endpoint was the time to hemostasis at the TBS following application of the hemostat. The secondary effectiveness endpoint was the proportion of patients with all treated bleeding sites achieving hemostasis within 3 min. The effectiveness endpoints were evaluated first based on non-inferiority to the control based on a per protocol population perspective, and if met, superiority was also tested from an intention to treat perspective.
Post-operative visits
Patients were assessed 24 hrs, 30 days, and 90 days post-surgery via a physical exam and measurement of both vital signs and clinical laboratory parameters. AEs were recorded until the end of the follow-up period. An SAE was defined as an AE that led to death or a serious deterioration in health (life-threatening illness or injury, permanent impairment, in-patient hospitalization, prolonged hospitalization, or required medical/surgical intervention to prevent life-threatening illness or injury). All AEs recorded as being possibly related to the device or whose relationship to the device was unable to be determined were later adjudicated for their device-relatedness by a single independent medical monitor who was blinded to the group assignments.
Statistical analysis
The required sample size to analyze effectiveness was based on a power of 80% and an alpha of 0.025, assuming true medians for time to hemostasis of Veriset™ hemostatic patch and TachoSil® as 0.5 min and 3.0 min, respectively. For effectiveness analyses, the required sample size was five patients per treatment group. For safety analyses, the required sample size was determined to provide a 90% probability of being able to detect an AE of a given type within the Veriset™ hemostatic patch group that occurs with a probability of at least 5%. Based on the specification, it was determined that 45 Veriset™ hemostatic patch patients were required. Using a 1:1 randomization scheme, a total sample size of 90 patients (45 per treatment group) was included.
Statistical analyses were performed and P-values were calculated from a two-sample t-test for continuous variables and Fisher’s exact test for categorical variables. All statistical analyses were performed using SAS® version 9.1 or higher. Two-sided tests and one-sided tests were performed at the 0.05 and 0.025 significance levels, respectively.
Results
Patient demographics
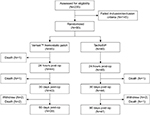
A total of 235 patients were analyzed for eligibility in the study (Figure 1). Of those, 145 failed the inclusion/exclusion criteria, 141 (97.2%) of whom did not have an appropriate TBS (bleeding not of Type 2 or 3 and/or it was not possible to hold pressure on the bleeding site for at least 3 min). The other four patients not randomized exhibited an appropriate TBS, but did not satisfy one or more of the criteria listed in Table 1. The remaining 90 patients were randomized 1:1 to receive treatment with either Veriset™ hemostatic patch or TachoSil®. Of the 45 patients randomized to the Veriset™ hemostatic patch treatment group, 39 completed the 90-day follow-up. Forty-one of the 45 patients randomized to the TachoSil® group completed their 90-day follow-up. All early withdrawals (two per treatment group) were by patient decision. The age and gender of patients included in this study were similar in both treatment groups, as well as baseline hemoglobin and platelet count (Table 2).
  | Table 2 Demographic and baseline characteristics (intent to treat population) Notes: Veriset™ (Covidien; Mansfield, MA, USA); TachoSil® (Nycomed; Zürich, Switzerland). |
Surgery
Patients included in the study underwent various types of cardiovascular procedures, with the most common being aortic surgery or CABG. There were no blood transfusions required due to bleeding from the TBS where the hemostat was applied, in either group (Table 3). Five patients from each treatment group did not undergo CPB, nine underwent aortofemoral bypass surgery and one underwent abdominal aorta bypass surgery. The selected TBS occurred from the aorta in 80% of patients in each treatment group (Table 4). Other sources of bleeding included the coronary artery, an atrium, ventricle, distal anastomosis, and diffuse bleeding from the operative field. Bleeding was of Type 2 in 68.2% of the Veriset™ hemostatic patch group and 84.4% of the TachoSil® group (p=0.0846), with the remaining patients exhibiting Type 3 bleeding (p=0.1337). There was no significant difference between the two treatment groups in any surgical procedure or TBS characteristic; however, there was a trend toward increased bleeding severity in the Veriset™ hemostatic patch group prior to hemostat application (p=0.0768). Conventional methods were used to obtain hemostasis first with suture/ligature used in over 50% of the cases, unless the investigator felt that use of these methods was not practical, which was the case in almost half of the patients in each group (40.9% of the Veriset™ hemostatic patch group and 42.2% of the TachoSil® group).
Safety outcomes
Safety was assessed at 24 hrs, 30 days, and 90 days after surgery. All AEs were recorded, along with their severity and relationship to the investigational device or control. A summary of AEs is shown in Table 5 and the safety endpoints of the study are shown in Table 6. Within 30 days post-surgery, 12/44 (27.3%) and 10/45 (22.2%) patients treated with Veriset™ hemostatic patch and TachoSil®, respectively, exhibited at least one SAE. The only SAEs to affect more than one patient treated with Veriset™ hemostatic patch were cardiac arrest, pleural effusion, and respiratory failure. None of the AEs in either treatment group were device-related and, within 5 days post-surgery, no patients required a reoperation for bleeding complications. Six patients died during follow-up (four from the Veriset™ hemostatic patch group and two from the TachoSil® group; p=0.4340), the causes of which were unrelated to the investigational device or control device.
Effectiveness outcomes
The median time to hemostasis at the TBS was 1.5 min in patients treated with Veriset™ hemostatic patch and 3.0 min in patients treated with TachoSil® (p<0.0001), which was the minimum time based on instructions for use (Table 7). This significant difference of p<0.001 remained when only patients on CPB were included in the analysis, although the median time to hemostasis at the TBS increased to 1.75 min with Veriset™ hemostatic patch. These results were statistically significant; clinical significance is determined by the clinician. The proportion of patients who achieved hemostasis at all treated bleeding sites at 3 min was similar between the two treatment groups. For the Veriset™ hemostatic patch, 49% achieved hemostasis at all treated bleeding sites within 1.5 min, 80% within 2 min, and 88% within 3 min. These percentages were slightly less when only patients on CPB were included at 47%, 78%, and 86%, respectively. A subgroup analysis of time to hemostasis at the TBS is shown based on hemostat application to the aorta (Table S1). Veriset™ hemostatic patch was slightly less effective when applied to the aorta than to other anatomical locations.
The results in Table 7 and Table S1 are shown on a per protocol population, which excluded four patients. One patient did not receive the study or control device after randomization, in two patients the study device was not applied to an appropriate TBS, and for one patient the study device was applied incorrectly. In the four cases in which Veriset™ hemostatic patch did not remain in place at the end of the procedure, there was no tissue damage observed after removal of the device (Table S2). Three device deficiencies occurred as a result of Veriset™ hemostatic patch being applied incorrectly, in which the device was not positioned correctly (PEG-side down, toward the bleeding site). In two of these patients, a new patch was applied, and hemostasis was obtained; rescue therapy was administered in the third patient. Only two device deficiencies occurred as a result of Veriset™ hemostatic patch not adhering to the TBS, both of which resulted in the use of rescue therapy. One patient treated with TachoSil® required rescue therapy due to hemostasis not being achieved, but TachoSil® was not removed prior to the administration of rescue therapy.
Discussion
This study tested the safety and effectiveness of a novel topical hemostat when applied to cardiac and vascular tissue during a variety of cardiovascular procedures, though the hemostat was applied to the aorta in the majority of patients included in the study. When applied per the manufacturer’s instructions for use, median time to hemostasis at the TBS was 3.0 min in patients treated with TachoSil® and 1.5 min in patients treated with Veriset™. The requirement in human studies to follow manufacturer instructions for use is a potential limitation of this randomized study as the minimum application time between the two products is not identical. TachoSil® labeling is based on regulatory approvals following three earlier clinical trials. Alternatively, Veriset™ hemostatic patch labeling only requires 30 s of pressure. A more direct comparison of time to hemostasis, in which the manufacturer instructions do not need to be adhered to, would require a nonclinical study. Although Veriset™ was found to be superior to TachoSil® in a previously published randomized clinical study of the liver,7 for the purposes of this study the two devices were determined to be equivalent.
Another potential study limitation is that CPB may have been managed differently across investigational sites, although baseline hemoglobin and platelet count were similar, and there was no differences in activated clotting time or the number of patients receiving protamine reversal prior or equal to the time of device application.
Based on the platform chemistry of the CE Mark-approved DuraSeal®, Veriset™ contains two primary components, an oxidized cellulose absorbable backing material and synthetic PEG. When in contact with physiological fluids Veriset™ forms a cross-linked PEG-based gel that rapidly absorbs fluid assisting in hemostasis while maintaining flexibility without the risk of viral transmission or use of the traditional coagulation cascade. Additionally, the oxidized cellulose may provide anti-microbial properties. Furthermore, Veriset™ hemostatic patch was shown to be safe, with no AEs resulting from use of the device. Results from this study support the inclusion of Veriset™ hemostatic patch as a new device in the cardiovascular surgeon’s toolkit.
A variety of hemostatic agents are already approved for use on cardiovascular tissue. Fibrin glues and sealants have been used for several decades.11 Prospective randomized controlled trials (RCTs) have shown approximately 90% of patients obtained hemostasis within 5 min of Tisseel® fibrin sealant (Baxter International Inc., Deerfield, IL, USA) application during major cardiovascular surgery.12,13 The safety and effectiveness of Gelfoam® sterile sponge (Pfizer, Inc., New York, NY, USA) and FloSeal™ hemostatic matrix (Baxter International Inc.) were compared in a randomized trial by Oz et al.14 At 3 min, roughly 31% of patients treated with FloSeal™ hemostatic matrix and 78% of patients treated with GelFoam® sterile sponge were still bleeding. TachoSil® has been shown previously in an RCT to obtain hemostasis faster (75% success rate at 3 min) than standard hemostatic fleece in cardiovascular indications.15 The current study showed a success rate of 88% within 3 min of applying Veriset™ hemostatic patch, a device that is completely free of animal- and human-derived components. Thus, results suggest that Veriset™ hemostatic patch is equivalent in efficacy to other products on the market, while eliminating components that have elicited safety concerns.16–20
Conclusion
This is the first study publishing on the safety and efficacy of Veriset™ hemostatic patch, which is completely free of animal- and human-derived components, in cardiovascular surgery applications, compared with a randomized TachoSil® control. Although there were differences in the minimum application time that affected the comparability of outcomes, the products were determined to be comparable. These data support the use of Veriset™ hemostatic patch during cardiovascular surgery, when perioperative bleeding requires urgent attention.
Acknowledgments
This work was sponsored and funded by Covidien (Mansfield, MA, USA), which contributed to the study design, data collection, and data analysis, and assisted with manuscript writing. Covidien LP is an indirect wholly owned subsidiary of Medtronic plc.
The authors would like to thank the other principal investigators who participated in the study: Professor Parla Astarci (Saint Luc Cliniques Universitaires), Professor Michael Schmoeckel (Asklepios Klinik St. Georg, Herzchirurgische Abteilung), Professor Martin Misfeld (Herzzentrum Leipzig), Professor Francis Wellens (UZ Brussels), Professor Filip Rega (UZ Leuven), and Michael Sudkamp, MD, PhD (Universitätsklinikum Freiburg). Additionally, they thank all the co-investigators and study personnel who assisted in the surgical procedures, follow-up assessments, and data collection. Statistical support was provided by Biostatistical Consulting, Inc. (Burlington, MA, USA). Editorial support was provided by John Hauschild and Nicholas Paquette (Medtronic).
Author contributions
Authors made substantial contributions to the study conduct and manuscript development beginning with the initial study conception, including study design (DG, MH, DK, PS, BV, TW, LH, MO), data acquisition (DG, MH, DK, PS, BV, TW, LH, MO), data analysis (DG, MH, DK, PS, BV, TW, LH, MO, CMR), data interpretation (DG, MH, DK, PS, BV, TW, LH, MO, CMR), manuscript writing (DG, CMR), critical revisions (DG, MH, DK, PS, BV, TW, LH, MO, CMR), final approval of the manuscript for submission (DG, MH, DK, PS, BV, TW, LH, MO, CMR); and agree to be accountable for the accuracy and integrity of the work (DG, MH, DK, PS, BV, TW, LH, MO, CMR).
Disclosure
All authors or their institutions received support from Covidien (the manufacturer of Veriset™) to conduct this study. The authors report no other conflicts of interest in this work.
References
Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727–729. | ||
Shander A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery. 2007;142(4 Suppl):S20–25. | ||
Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med. 2004;30(10):1873–1881. | ||
Biancari F, Airaksinen KE, Lip GY. Benefits and risks of using clopidogrel before coronary artery bypass surgery: systematic review and meta-analysis of randomized trials and observational studies. J Thorac Cardiovasc Surg. 2012;143(3):665–675. | ||
Dacey LJ, Munoz JJ, Baribeau YR, et al. Reexploration for hemorrhage following coronary artery bypass grafting: incidence and risk factors. Northern New England Cardiovascular Disease Study Group. Arch Surg. 1998;133(4):442–447. | ||
Shander A, Kaplan LJ, Harris MT, et al. Topical hemostatic therapy in surgery: bridging the knowledge and practice gap. J Am Coll Surg. 2014;219(3):570–579.e4. | ||
Ollinger R, Mihaljevic AL, Schuhmacher C, et al. A multicentre, randomized clinical trial comparing the Veriset haemostatic patch with fibrin sealant for the management of bleeding during hepatic surgery. HPB (Oxford). 2013;15(7):548–558. | ||
Schuhmacher C, Pratschke J, Weiss S, et al. Safety and effectiveness of a synthetic hemostatic patch for intraoperative soft tissue bleeding. Med Devices (Auckl). 2015;8:167–174. | ||
Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. | ||
Howk K, Fortier J, Poston R. A novel hemostatic patch that stops bleeding in cardiovascular and peripheral vascular procedures. Ann Vasc Surg. 2016;31:186–195. | ||
Rousou JA. Use of fibrin sealants in cardiovascular surgery: a systematic review. J Card Surg. 2013;28(3):238–247. | ||
Lowe J, Luber J, Levitsky S, et al. Evaluation of the topical hemostatic efficacy and safety of TISSEEL VH S/D fibrin sealant compared with currently licensed TISSEEL VH in patients undergoing cardiac surgery: a phase 3, randomized, double-blind clinical study. J Cardiovasc Surg (Torino). 2007;48(3):323–331. | ||
Rousou J, Levitsky S, Gonzalez-Lavin L, et al. Randomized clinical trial of fibrin sealant in patients undergoing resternotomy or reoperation after cardiac operations. A multicenter study. J Thorac Cardiovasc Surg. 1989;97(2):194–203. | ||
Oz MC, Cosgrove DM 3rd, Badduke BR, Hill JD, Flannery MR, Palumbo R, Topic N. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. The Fusion Matrix Study Group. Ann Thorac Surg. 2000;69(5):1376–1382. | ||
Maisano F, Kjaergard HK, Bauernschmitt R, et al. TachoSil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: a randomised controlled trial. Eur J Cardiothorac Surg. 2009;36(4):708–714. | ||
Hino M, Ishiko O, Honda KI, Yamane T, Ohta K, Takubo T, Tatsumi N. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000;108(1):194–195. | ||
Kawamura M, Sawafuji M, Watanabe M, Horinouchi H, Kobayashi K. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg. 2002;73(4):1098–1100. | ||
Pope M, Johnston KW. Anaphylaxis after thrombin injection of a femoral pseudoaneurysm: recommendations for prevention. J Vasc Surg. 2000;32(1):190–191. | ||
Tadokoro K, Ohtoshi T, Takafuji S, et al. Topical thrombin-induced IgE-mediated anaphylaxis: RAST analysis and skin test studies. J Allergy Clin Immunol. 1991;88(4):620–629. | ||
Wai Y, Tsui V, Peng Z, Richardson R, Oreopoulos D, Tarlo SM. Anaphylaxis from topical bovine thrombin (Thrombostat) during haemodialysis and evaluation of sensitization among a dialysis population. Clin Exp Allergy. 2003;33(12):1730–1734. |
Supplementary materials
  | Table S3 Independent ethics committees and their associated clinical trial sites |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.