Back to Journals » Cancer Management and Research » Volume 11
A quantified risk-scoring system including the visceral fat area for peritoneal metastasis of gastric cancer
Authors Chen X, Chen W, Huang Y, Xu J , Zeng Y, Shi M, Xu L, Zhang W, Zhu G, Mao C , Shen X
Received 13 November 2018
Accepted for publication 26 February 2019
Published 10 April 2019 Volume 2019:11 Pages 2903—2913
DOI https://doi.org/10.2147/CMAR.S194356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Xiaodong Chen,1,* Weisheng Chen,2,* Yunshi Huang,2 Jingxuan Xu,1 Yunpeng Zeng,1 Mingming Shi,2 Libin Xu,2 Weiteng Zhang,2 Guanbao Zhu,2 Chenchen Mao,1 Xian Shen1,2
1Department of Gastrointestinal Surgery, The Second Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2Department of Gastrointestinal Surgery, The First Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Background: Peritoneal metastases of gastric cancer are usually detected using imaging, However, the results of imaging modalities are not always reliable; therefore, the prediction of prognosis based on these findings is therefore inaccurate. As visceral obesity has been identified as a potential risk factor for cancer, the present study aimed to evaluate the predictive value of visceral fat area (VFA), a representative marker of visceral obesity, for peritoneal metastasis in patients with gastric cancer and to construct a reliable preoperative prediction system for peritoneal metastasis.
Patients and methods: We enrolled 859 patients with gastric cancer. The VFA and other objective clinical tumor characteristics were evaluated using receiver operating characteristic (ROC) curves. Independent predictors of peritoneal metastasis were determined using logistic regression analysis; a prediction system was also evaluated using ROC curves.
Results: The ROC curves indicated a VFA cutoff value of 91.00 cm2 as predictive of peritoneal metastasis. On logistic regression, visceral obesity (VFA ≥91.00 cm2) was identified as an independent predictor of peritoneal metastasis, with an area under the ROC curve of 0.659; the platelet-to-lymphocyte ratio (PLR), invasion depth, and vascular invasion were also identified as independent predictors. On integrating these predictors into a single prediction system, peritoneal metastases were more reliably predicted (area under the ROC curve=0.779).
Conclusions: Visceral obesity, as defined by the VFA, effectively predicted peritoneal metastases in our cohort. Our scoring system may be a reliable instrument for identifying patients with peritoneal metastasis.
Keywords: gastric cancer, peritoneal metastasis, visceral fat area, risk-scoring system
Introduction
Gastric cancer (GC) is the fourth most common cancer worldwide, and the fourth and second most common cause of cancer-related mortality in the world1 and China,2 respectively. Peritoneal metastasis is common in GC, and contributes to poor prognosis; it is therefore an indispensable indicator of prognosis.3,4 A previous study demonstrated that peritoneal metastases accounted for 50% of all GC-related deaths.4 The occurrence of peritoneal metastasis also influences treatment options, usually making complete resections (R0) less feasible. Neoadjuvant chemotherapy5 and conversion therapy6 is usually more suitable for these patients.
Currently, peritoneal metastases are usually detected using imaging techniques such as ultrasonography and computed tomography (CT). However, peritoneal metastases may be undetectable, and therefore these imaging modalities do not always provide reliable diagnoses or accurate prognostic predictions. Although positron emission tomography (PET)-CT has a higher predictive value in diagnosing peritoneal metastasis compared to ultrasonography and CT,7,8 the high associated costs greatly limit its use. Therefore, accurate and affordable diagnostic methods are urgently needed.
Obesity, a risk factor for many different human cancers including those of the breast,9 colon10 and other sites;11 it is reaching epidemic proportions in the United States,12 where it affects more than one third of the population. Obesity has increasingly attracted attention in the medical field, owing to a higher risk of postoperative complications in this population. Additionally, hypertrophied adipose tissue deposits in obese patients were found to secrete adipokines and cytokines, which promote adhesions, and migration and invasion of tumor cells.13
Visceral obesity (VO), defined as a high visceral fat area (VFA), is an indicator of obesity, and has been demonstrated to be more strongly associated with incisional hernia after colorectal surgery than an elevated body mass index (BMI).14 We had demonstrated in a previous study that the VFA was a better predictor of postsurgical gastroparesis syndrome compared to BMI.15 Therefore, we hypothesized that the VFA may also be a useful indicator of the risk of peritoneal metastasis. In this study, we aimed to determine the relationship between the incidence of peritoneal metastases from GC and VFA. We also aimed to construct a more useful scoring system based on independent associated factors that would improve the accuracy of preoperative diagnoses of peritoneal metastases.
Patients and methods
Patients
Data were prospectively collected from 859 patients with GC who underwent subtotal gastrectomy at the Gastrointestinal Surgical Department, Second Affiliated Hospital of Wenzhou Medical University and the First Affiliated Hospital of Wenzhou Medical University in China between January 2009 and December 2012. Patients who lacked imaging data were excluded. Written informed consent was obtained from every participant enrolled in the study. Current study was carried out in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committees of the Second Affiliated Hospital of Wenzhou Medical University and the First Affiliated Hospital of Wenzhou Medical University.
Diagnosis of peritoneal metastasis
Peritoneal metastases were diagnosed according to the criteria of the Japanese Gastric Cancer Treatment Guidelines (15th edition).16 According to this guideline, metastases limited to the greater and lesser omentum, anterior lobe of the transverse mesocolon, pancreatic capsule, spleen, and to the upper abdominal peritoneum (visceral peritoneum above the transverse plane and parietal peritoneum above the umbilicus) are considered to be peritoneal metastases. Peritoneal metastases were diagnosed from intraoperative frozen sections and postoperative diagnostic pathology.
Measurement of VFA
Preoperatively, all patients underwent CT of the whole abdomen. The VFA was calculated with an accuracy of 99% by measuring the total abdominal fat area in a cross-section at the level of the umbilicus. The Hounsfield scale was used to distinguish adipose tissue from other tissues. In this study, adipose tissue was defined to be within the range of −140 to −50 Hounsfield units (HU). The total fat area was calculated using a dedicated processing system (version 3.0.11.3, BN17 32-bit; INFINITT Healthcare Co., Ltd., Seoul, South Korea).
Cutoff point for VFA
We determined the cutoff point for VFA as the maximal Youden index value on the receiver operating characteristics (ROC) curve. Patients were then divided into two groups based on this cutoff point, namely, the VO and control groups.
Statistical analysis
The Kolmogorov–Smirnov test was performed to assess the distribution equality of the continuous parameters. The normally distributed data are presented as means ± standard deviations, whereas non-normally distributed data are presented as medians and interquartile ranges (IQRs). On univariate analyses, the independent t- and Mann–Whitney U-tests were used to analyze inter-group differences among continuous variables, while the chi-square and Fisher’s exact tests were applied to categorical variables. Based on the results of these univariate analyses, a multivariate logistic regression analysis was performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) of the independent variables. The area under the ROC curve (AUC) was used to compare the accuracy of our scoring system with that of the individual clinicopathologic characteristics. P-values <0.05 were considered statistically significant. All statistical analyses were performed using the SPSS software package (version 22.0; SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
As imaging data was available for all the 859 patients treated, they were all enrolled in the study. As shown in Table 1, 672 of the 859 enrolled patients were males (78.2%), and the overall median age was 63.6 years (IQR=56.0–72.0 years). The median BMI and VFA was 21.8 kg/cm2 (IQR=19.6–23.7 kg/cm2) and 89.6 cm2 (IQR=39.2–124.9 cm2), respectively. The tumors were located in the antrum in a majority of patients (n=579, 67.4%). Lymphatic and vascular invasion was noted in 508 and 337 patients, respectively. A large proportion of patients (n=671, 78.1%) had a differentiated tumor on histopathology, and 87 patients had detectable peritoneal metastases.
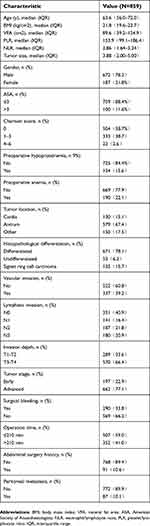 | Table 1 Clinical and pathological characteristics |
Preoperative characteristics
As shown in Figure 1 and Table 2, compared to those without peritoneal metastases, patients with peritoneal metastases from GC had a significantly higher PLR (P<0.001) and VFA (P<0.001) and significantly lower neutrophil-to-lymphocyte ratio (NLR) (P=0.001). These two groups did not differ significantly in terms of age and BMI. An analysis of the AUCs yielded high values for PLR (0.610, 95% CI =0.551–0.669), VFA (0.659, 95% CI =0.604–0.714), and NLR (0.591, 95% CI =0.528–0.654), indicating that these factors are individually powerful predictors of peritoneal metastasis (Figure 2A).
 | Table 2 General condition, according to peritoneal metastasis involvement |
 | Figure 1 Distribution of VFA betweenPMPG and PMNG.Abbreviations: VFA, visceral fat area; PMPG, peritoneal metastasis positive group; PMNG, peritoneal metastasis negative group. |
Correlation of clinicopathologic characteristics with VO
From the ROC curve analysis, the VFA cutoff value for peritoneal metastasis was determined to be 91.00 cm2 (Figure 2A), with a sensitivity and specificity of 70.1% and 60.7%, respectively. Using this cutoff value, 374 (43.5%) patients were classified as viscerally obese (VFA ≥91.00 cm/m2). VO was found to be significantly associated with relatively high Charlson comorbidity scores (P<0.001), high American Society of Anesthesiologists (ASA) stages (P=0.0.025), low PLR (P=0.013), and long operation times (P=0.004) (Table 3).
 | Table 3 Clinicopathologic features of patients, based on the VFA |
Univariate and multivariate analyses of variables associated with peritoneal metastasis
The chi-square test was subsequently used to evaluate the relationship of clinical characteristics with peritoneal metastasis. Univariate analysis identified the VFA (P<0.001), Charlson score (P=0.016), PLR (P<0.001), NLR (P=0.001), tumor size (P<0.001), vascular invasion (P<0.001), lymphatic invasion (P=0.004), invasion depth (P<0.001), tumor stage (P<0.001), and operation time (P=0.032) to be significantly correlated with peritoneal metastasis (Table 4). These factors were subsequently included in the multivariate logistic regression analysis to identify the independent risk factors for peritoneal metastasis. As shown in Table 5, PLR (OR=2.197, P=0.001), invasion depth (OR=4.330, P=0.009 for T2; OR=4.489, P=0.009 for T3), vascular invasion (OR=2.328, P=0.002), and VFA (OR=4.027, P<0.001) independently predicted the presence of peritoneal metastasis.
 | Table 4 Univariate analysis of the risk of peritoneal metastasis |
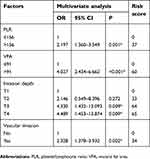 | Table 5 Multivariate analysis to evaluate potential predictive factors for peritoneal metastasis and the scoring of these factors |
Derivation of the scoring system
We then used the independent risk factors identified on multivariate logistic regression analysis to establish a scoring system for the accurate prediction of peritoneal metastasis. To establish this, we calculated a risk score for each identified factor via logarithmic transformation and multiplication by 100 (Table 5) and summed these values to determine the combined score. The ROC analysis revealed that the combined score had a better predictive accuracy for peritoneal metastasis (ie, a larger AUC), compared with the individual scores (AUCs: combined score, 0.779, 95% CI=0.735–0.824; PLR, 0.600, 95%CI =0.536–0.664; T3, 0.538, 95%CI =0.472–0.603; T4, 0.592, 95%CI =0.529–0.655; vascular invasion, 0.659, 95%CI =0.599–0.719; and VFA, 0.696, 95% CI=0.612–0.781) (Figure 2B).
Discussion
Peritoneal metastases from GC are usually associated with poor prognosis.3 As patients with peritoneal metastasis may not benefit from gastrectomy and regional lymphadenectomy alone, accurate preoperative diagnosis is essential in determining optimal treatment using an individualized treatment strategy.
According to a recent study, the global overweight or obese population has surpassed the underweight population in numbers for the first time.17 Notably, China has the largest number of overweight people (more than 89.6 million in 2014).17 In the literature, obesity is usually determined using the BMI, which is easily calculated. However, in this study we used the VFA, which is a novel factor reflecting the body fat composition. In our cohort, patients with peritoneal metastases from GC had a significantly higher VFA relative to patients without metastasis. However, the two groups did not differ in terms of BMI. This finding was consistent with our previous observation that VFA was superior to BMI as an obesity index predictive of the risk of post- operative complications in those undergoing abdominal surgery (eg, postsurgical gastroparesis syndrome).15 Based on these findings, we aimed to determine the relationship between VO and peritoneal metastases.
The general lack of consensus regarding the definition of VO made it difficult to choose a VFA cutoff value in our study. Using ROC curve analysis, we defined VO using a VFA cutoff value of 91 cm2, which was similar to the ≥94 cm2 value we had used to define VO in our previous work.15 Using this cutoff, 43.5% of patients in our cohort were found to have VO. Our study is the first to demonstrate that these patients with undetected obesity are more likely to develop peritoneal metastasis, compared to their non-obese counterparts. We further confirmed VO to be an independent risk factor for peritoneal metastases on multivariate logistic regression analysis.
Pathophysiologically, the link between VO and an increased risk of peritoneal metastasis may be best explained by the adipokine-cancer and insulin-cancer hypotheses. In an obese person, the adipose tissue is thought to remain in a state of low-grade chronic inflammation. This tissue generates reactive oxygen species (ROS) with mitogenic and potentially tumorigenic constituents in low concentrations.18,19 Additionally, in a comparison of visceral and subcutaneous adipose tissue volumes, a greater adipose tissue mass or volume was found to correlate with higher serum levels of pro-inflammatory cytokines.20 This chronic elevation of systemic pro-inflammatory cytokines and ROS levels may promote tumorigenesis and tumor progression.20
It is known that patients with metabolic syndrome have a higher incidence of cancer.21 Accumulating evidence also suggests that insulin resistance and the metabolic effects associated with obesity are risk factors for cancer development. In patients with insulin resistance, reduced tissue sensitivity to insulin results in hyperglycemia and hyperinsulinemia. Consequently, chronic hyperinsulinemia promotes the secretion of insulin-like growth factor (IGF)-1 and reduces the production of IGF binding proteins, which further increases the circulating levels of IGF. Through its receptor, IGF activates downstream signaling pathways with mitogenic, proangiogenic, and anti-apoptotic effects,22 all of which enhance cell proliferation and promote tumor development and metastasis.
In contrast to the expected results, we observed that viscerally obese patients had a significantly lower PLR, compared to non-obese patients, whereas no statistical correlation was observed between VO and NLR. However, consistent with our previous results,23 we identified PLR to be an independent risk factor for peritoneal metastases. This suggests that adipokine-related cancers may occur as a result of chronic inflammation due to local inflammatory cells, rather than by systemic inflammation. However, further studies are needed to explore this mechanism in detail.
In our cohort, patients with VO were older than their non-VO counterparts. This is consistent with previous observations that the body fat content increases with age.24 It also demonstrates that this relative increase in fat greatly reduces insulin sensitivity.25 In addition to age, we investigated the significance of several tumor characteristics, such as tumor size, histopathological differentiation, vascular invasion, lymphatic invasion, and depth of invasion, to improve the diagnostic accuracy of our scoring system for peritoneal metastases.
Although the initial univariate analysis identified all factors except histopathological differentiation to be associated with peritoneal metastasis, subsequent multivariate analysis identified only vascular invasion and depth of invasion to be independent risk factors for this condition. This implies that more invasive tumors are more likely to metastasize, as vascular invasion and serosal breakthrough would enable tumor cells to travel through the circulation or be directly implanted on the peritoneum.
Finally, we designed a predictive scoring system that collectively considered the VO, PLR, invasion depth, and vascular invasion in each case, and performed a combined ROC analysis to determine whether this system had improved the diagnostic accuracy for peritoneal metastasis. The combined score demonstrated an AUC of 0.779, with higher sensitivity (75.9%) and specificity (68.0%), compared to the individual scores. These findings therefore concluded that this preoperative scoring system could be useful for diagnosing peritoneal metastasis.
Several researchers had previously constructed systems to predict peritoneal metastasis. One group reported a specificity and sensitivity of 78.3% and 88.5%, respectively, for a predictive equation including tumor size, tumor stage, lymph node invasion, and histological differentiation as predictor variables.26 However, as all the variables were tumor-specific, the equation did not reflect the patient’s general condition. Although another study27 successfully used a combination of the levels of lysyl oxidase, carcinoembryonic antigen, carbohydrate antigen (CA) 724, CA-199, and CA-125 to predict peritoneal metastasis with a sensitivity of 91.30%, the application of this system was hindered by the cost and rarity of lysyl oxidase detection. Although the results of our present and previous studies are similar,23 the present study was the first to investigate the relationship between VO and peritoneal metastasis. Our results indicate that VO is an indispensable predictor of peritoneal metastasis.
Despite the useful findings, the present study had some notable limitations. First, it included a small sample size, as only 87 patients were diagnosed with peritoneal metastasis. Second, although ROC curves are appropriate for establishing VO cutoff values, a standardized cutoff value is not available. Finally, this was a retrospective study. This scoring system should therefore be tested further in a prospective study, which should include prognostic analysis.
Conclusion
This study is the first to determine the relationship between VO and peritoneal metastasis from GC. VO was found to be an independent and significant predictor of peritoneal metastasis. A scoring system combining VO, PLR, invasion depth, and vascular invasion status considerably improved the accuracy in predicting peritoneal metastasis, compared to each factor alone. This economical and convenient new scoring system may be a useful tool in predicting peritoneal metastasis in the clinic. Moreover, it is likely to play an important role in individualizing treatment for patients with GC having no metastases on imaging.
Abbreviation list
GC, gastric cancer; ASA, American society of anesthesiologists; AUC, area under curve; BMI, body mass index; CI, confidence interval; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; NRS, nutritional risk screening; OR, odds ratio; ROC, receiver operating characteristic; VO, visceral obesity; VFA, visceral fat area.
Ethics statement
Ethical approval was obtained from the ethics committee of The First Affiliated Hospital of Wenzhou Medical University and The Second Affiliated Hospital of Wenzhou Medical University, and written informed consent was obtained from the patients whose tissue and medical data were used in this study.
Availability of data and materials
The datasets used during the current study shall be available from the corresponding author on request.
Acknowledgments
The authors thank all the participants in this study and the members of our research team. This study was funded by the Department of Health of Zhejiang Province, China (grant no. 2016DTA006) and the Wenzhou Municipal Science and Technology Bureau (grant no. Y20150057). There are no other commercial interests or sources of financial or material support to declare.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
3. Ahmed A, Ukwenya AY, Makama JG, Mohammad I. Management and outcome of gastric carcinoma in Zaria, Nigeria. Afr Health Sci. 2011;11:353–361.
4. Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–357. doi:10.1046/j.1365-2168.2000.01358.x
5. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi:10.1016/S0140-6736(16)30354-3
6. Sato Y, Ohnuma H, Nobuoka T, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017;20:517–526. doi:10.1007/s10120-016-0633-1
7. Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol. 2011;11:19. doi:10.1186/1471-230X-11-93
8. Ozkan E, Araz M, Soydal C, Kucuk ON. The role of 18F-FDG-PET/CT in the preoperative staging and posttherapy follow up of gastric cancer: comparison with spiral CT. World J Surg Oncol. 2011;9:75. doi:10.1186/1477-7819-9-75
9. Yoo K, Tajima K, Park S, et al. Postmenopausal obesity as a breast cancer risk factor according to estrogen and progesterone receptor status (Japan). Cancer Lett. 2001;167:57–63.
10. Juo YY, Gibbons MAM, Dutson E, et al. Obesity is associated with early onset of gastrointestinal cancers in California. J Obes. 2018;2018:7014073. doi:10.1155/2018/7014073
11. Tanaka K, Tsuji I, Tamakoshi A, et al. Obesity and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:212–221. doi:10.1093/jjco/hyr198
12. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi:10.1001/jama.2014.732
13. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi:10.1016/j.bbalip.2013.02.010
14. Aquina CT, Rickles AS, Probst CP, et al. Visceral obesity, not elevated BMI, is strongly associated with incisional hernia after colorectal surgery. Dis Colon Rectum. 2015;58:220–227. doi:10.1097/DCR.0000000000000261
15. Chen XD, Mao CC, Zhang WT, et al. A quantified risk-scoring system and rating model for postsurgical gastroparesis syndrome in gastric cancer patients. J Surg Oncol. 2017;116:533–544. doi:10.1002/jso.24691
16.
17. Collaboration NCDRF Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi:10.1016/S0140-6736(16)30054-X
18. Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)–induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–173. doi:10.1016/j.mrfmmm.2011.02.015
19. Kim YJ, Kim EH, Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. 2012;27:1004–1010. doi:10.1111/j.1440-1746.2012.07108.x
20. Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi:10.1161/CIRCULATIONAHA.107.710509
21. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi:10.1146/annurev.med.080708.082713
22. Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi:10.1210/er.2006-0001
23. Chen XD, Mao CC, Wu RS, et al. Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PLoS One. 2017;12:e0175074. doi:10.1371/journal.pone.0175074
24. Reinders I, Visser M, Schaap L. Body weight and body composition in old age and their relationship with frailty. Curr Opin Clin Nutr Metab Care. 2017;20:11–15. doi:10.1097/MCO.0000000000000332
25. Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi:10.1016/j.biochi.2015.10.024
26. Kurita N, Shimada M, Utsunomiya T, et al. Predictive factors of peritoneal metastasis in gastric cancer. Hepatogastroenterology. 2010;57:980–983.
27. Lai H, Jin Q, Lin Y, et al. Combined use of lysyl oxidase, carcino-embryonic antigen, and carbohydrate antigens improves the sensitivity of biomarkers in predicting lymph node metastasis and peritoneal metastasis in gastric cancer. Tumour Biol. 2014;35:10547–10554. doi:10.1007/s13277-014-2355-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

