Back to Journals » Journal of Inflammation Research » Volume 14
A Prospective Clinical Study on MGMT Protein Expression and the Effect of Gene Promoter Methylation on Sensitivity to Chemotherapeutics in Spinal Glioma
Authors Sun P, Fan DJ, Fan T, Li X, Qi XL, Zhao XG, Gai QF
Received 25 May 2021
Accepted for publication 28 August 2021
Published 18 September 2021 Volume 2021:14 Pages 4777—4784
DOI https://doi.org/10.2147/JIR.S321790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Peng Sun,1,* Duo-Jiao Fan,1,* Tao Fan,2 Xin Li,3 Xue-Ling Qi,2 Xin-Gang Zhao,2 Qi-Fei Gai2
1Baoding Second Hospital, Hebei, 071051, People’s Republic of China; 2Department of Neurosurgery Spine Center, Sanbo Brain Hospital, Capital Medical University, Beijing, 100093, People’s Republic of China; 3Department of Neurosurgery, Baoding No. 1 Central Hospital, Hebei, 071051, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tao Fan
Department of Neurosurgery Spine Center, Sanbo Brain Hospital, Capital Medical University, No. 50 Yikesong Road, Xiangshan, Haidian District, Beijing, 100093, People’s Republic of China
Tel +86 010 62856746
Fax +86 010 62856902
Email [email protected]
Objective: The present study discusses the O6-methylguanine-DNA methyltransferase (MGMT) protein expression of spinal glioma cells and the correlation between the sensitivity of promoter methylation of the MGMT gene to chemotherapy drugs, establishes a prediction method for the sensitivity of chemotherapy drugs on spinal gliomas, providing a theoretical basis for determining the best chemotherapy regimens for clinical patients after a spinal glioma operation.
Methods: A total of 67 patients, who received microsurgical resection for spinal glioma from October 2010 to June 2016, were selected for the present study. Immunohistochemistry and methylation were performed after the operation. Among these patients, 47 patients with postoperative chemotherapy were assigned as the experimental group, while 20 patients without chemotherapy were designated as the control group.
Results: Among the 47 patients in the experimental group, 39 patients had no tumor recurrence after two years, while tumors increased and symptoms were aggravated in eight patients. The progression-free survival rate of chemotherapy was 82.9%, and the two-year survival rate was 100%. The adverse reactions of patients during chemotherapy were slight. Among the 20 patients in the control group, seven patients had no tumor recurrence, while 13 patients had increased tumor size, and the progression-free survival rate was 35.0%.
Conclusion: Under the guidance of MGMT immunohistochemical detection and MGMT gene promoter methylation detection after surgery, chemotherapy can effectively delay tumor recurrence, prevent a reoperation, and have good safety and tolerability. This chemotherapy regimen has good prospects.
Keywords: spinal cord, glioma, microscopic surgery, chemotherapy, temozolomide
Introduction
Spinal gliomas are the most common intramedullary tumors of the spinal cord, which occur in the ectodermal layer of the nervous system of the human spine, and account for approximately 80% of intramedullary tumors of the spinal cord.1 Spinal gliomas include ependymomas, astrocytomas, and less frequent multiform glioblastomas, oligodendrogliomas, and mixed gliomas.1–25 Ependymomas and astrocytomas are the most common forms of spinal gliomas. Clinically, spinal gliomas are far less frequent than brain gliomas.2 However, due to the particularity of its location, high disability rate, and great harm to patients, it is worth further study.2,3
Spinal gliomas are a cluster of serious diseases that threaten human health with low incidence but high disability rates.4 The majority of neurosurgeons have reported few cases in clinical practice, thereby making it relatively difficult to handle.4 However, with the development of imaging diagnosis, microsurgery, and intraoperative electrophysiological monitoring, the resection rate of spinal gliomas has been dramatically improved.5 Meanwhile, since its pathological classification belongs to malignant tumors, its recurrence after surgery needs to be treated through an operation again, bringing great pain to patients.6 There has been a recent genome-wide in vivo screen identifying drivers of spinal astrocytomas and showing EGFR and PTEN can initiate spinal astrocytomas in mice (Noorani et al 2020, Genome Biology, PiggyBac mutagenesis, and exome sequencing identify genetic drivers of EGFR-mutant gliomas).7 The DNA of O6-methylguanine-DNA methyltransferase (MGMT) repairs enzymes. It is located on the long arm of human chromosome 10, and its primary function is to repair damaged guanine nucleotides by transferring methyl groups at the guanine O6 site to cysteine residues, thus avoiding alkylating agent-induced genetic mutations, cell death, and tumorigenesis. Therefore, the MGMT gene is a factor that causes resistance to gene chemotherapy. In order to delay the recurrence and improve the patient’s quality of life, postoperative radiotherapy and chemotherapy have undoubtedly become the essential means for therapy of this disease after surgery.6 As a highly efficient DNA repairing enzyme, the role of MGMT in the body is to protect cells from damage induced by alkylating agents that are widely present in the environment.7 Cisplatin (DDP) is a non-specific cell cycle antineoplastic drug.8 It mainly acts on the purine and pyrimidine bases of DNA and inhibits DNA replication and RNA and protein synthesis in tumor cells.8 The main side effects of DDP in chemotherapy are bone marrow inhibition, gastrointestinal discomfort, and hepatic and renal toxicity.9 Recently, many studies have demonstrated that the combined chemotherapeutic protocol of temozolomide (TMZ) and DDP might target those with the positive expression of the DNA repairing gene MGMT and have a good therapeutic effect.10,11
Materials and Methods
Clinical Materials
A total of 67 patients with spinal glioma, who underwent microsurgical resection from October 2010 to June 2016 at the Fifth Ward of Neurosurgery, Beijing Sanbo Brain Hospital, were included in the present study. The tumor tissues obtained from all patients were diagnosed as spinal glioma by routine histopathological examination at the Department of Pathology, Beijing Sanbo Brain Hospital. The flow of patients is shown in Figure 1. The Ethics Committee of our hospital approved the current study, and all patients signed written informed consent.
 |
Figure 1 The flow of patients. |
Patients were selected for chemotherapy or control if they met all the following inclusion criteria: (1) patients were ≥18 years old on the day of enrollment; (2) patients had histologically-confirmed astrocytoma (grade III) or ependymoma (grade III), according to the World Health Organization classification; (3) prior to chemotherapy, the patients’ white blood cell count was ≥4.0 × 109/L, the platelet count was ≥100 × 109/L, and hemoglobin was ≥100 g/L; (4) patients did not have severe hepatic or renal dysfunction; (5) patients did not receive radiotherapy after surgery. The population for analysis is intention-to-treat.
These patients were divided into two groups: the experimental and control groups. (1) Experimental group: a total of 47 patients who agreed and completed chemotherapy were assigned to the experimental group. These patients used alkylating agents during chemotherapy. Patients with negative MGMT expression and gene promoter methylation were orally given TMZ as chemotherapy based on the immunohistochemistry and molecular pathology results. TMZ in combination with DDP was given to patients with positive MGMT protein expression or without methylation. (2) Control group: a total of 20 patients underwent microsurgery and refused to receive radiotherapy and chemotherapy after surgery.
The MGMT protein expression of the tumor was determined by immunohistochemistry and methylation analysis after surgery for patients in the experimental group, and these were all performed at the Department of Pathology, Beijing Sanbo Brain Hospital.
Detection of MGMT Protein by Immunohistochemistry
The steps of the immunohistochemistry were as follows: immobilization, dehydration, transparency, wax immersion, and embedding of specimens. Then, the specimens were cut into sections with a thickness of 5 µm. Subsequently, immunohistochemical staining was performed using the Streptomyces antibiotic protein-peroxidase ligation (SP) method to detect the MGMT protein expression.
Judging criteria for the MGMT results: under the 400-fold field of vision of optical microscopy, the dense areas of primary spinal glioma cells were observed in five fields, and a total of 1000 cells were counted. Then, the rates of positive cells were respectively calculated.
The nucleus and cytoplasm of glioma cells that contained brown-yellow granules were considered positive and graded according to the following criteria and based on the positive cell rate and staining intensity:
(1) Strong positivity (++): the rate of positive cells ranged from 25 to 50% in the random field of vision (Figure 2A);
 |
Figure 2 O6-methylguanine-DNA methyltransferase (MGMT) immunohistochemistry of the patient. (A) strong positivity (++); (B) positivity (+); (C) negativity (–). |
(2) Positivity (+): the rate of positive cells ranged from 10 to 25% in the random field of vision (Figure 2B);
(3) Negativity (–): no brown-yellow sediment particles or the rate of positive cells was less than 10% in the random field of vision (Figure 2C).
Detection of MGMT Methylation
The DNA of tissues in a white slice was extracted using a paraffin-embedded tissue DNA rapid extraction kit, and the concentration and purity of DNA were determined. DNA sulphuration was performed using an EZ DNA Methylation™ Kit (according to kit instructions).
The design of the MGMT gene primer sequence was as follows: IntMe-S: TTTCGACGTTCGTAGGTTTTCGC; IntMe-AS: GCACTCTTCCGAAAACGAAACG; IntUn-S: TTTGTGTTTTGATGTTTGTAGGTTTTTGT; ntUn-AS: AACTCCACACTCTTCCAAAAACAAAACA.
The PCR system: 10 × buffer: 2.5 μL; 10 mM of dNTP: 2.5 μL; 10 P (primer 1/2): 1 μL; Taq enzyme: 0.1 μL; ddH2O: 16.9 μL; Sulfurized DNA: 2 μL.
The setting of the PCR instrument: (95°C, 15 minutes)→(95°C, one minute, 60°C, 30 seconds, 72°C, 30 minutes) 35 cycles→(72°C, seven minutes; 4°C, ∞).
After electrophoresis through 2% agarose gel, a UV Gel automatic imager was used to detect the results.
Chemotherapy Administration Method
The 47 patients in the experimental group were given chemotherapy according to the MGMT expression results determined by immunohistochemistry and MGMT methylation. TMZ was orally administered as chemotherapy in patients with negative MGMT expression and gene promoter methylation. The dosage of TMZ (Finland Olian Company, 20 mg, trade name “Taidao”; Jiangsu Tianshili Company, 50 mg, trade name “Tiqing”) was calculated according to the patient’s body surface area. The equation to determine the body surface area was as follows: body surface area (m2) = (height + weight – 60)/100. The dosage of TMZ was 150–200 mg/m2, which was according to the patient’s body surface area. Furthermore, the daily TMZ dosage = 200 mg/m2 × body surface area. The dosage was 150–200 mg·m-2·d-1, d1-5, which was repeated every four weeks and administered six times in total. TMZ in combination with DDP was administered as chemotherapy to patients with positive MGMT protein expression or without methylation. First, DDP was given to decrease the activity of MGMT protein in tumor cells and increase the clinical effects of the alkylating agent. The details are as follows: DDP intravenously guttae 40–50 mg·m-2·d-1, d1-2. Due to the serious side effects of DDP, especially in females during chemotherapy, a 500-mL solution was used to dissolve the DDP, and a 1000 mL glucose solution (5%) or natural saline solution was intravenously given to expand the fluid volume. Then, 5 mg of Tropisetron, as an antiemetic, and 250 mL of 20% mannitol were administered intravenously. Afterward, 1000 mL of glucose solution (5%) or natural saline solution was intravenously given after DDP administration, and 5 mg of Tropisetron was intravenously administered after six hours. Routine blood tests were performed, and hepatic and renal function was tested two days after DDP usage. Then, TMZ was given at 150–200 mg·m-2·d-1, d2-6, which was repeated every four weeks and administered six times in total. The chemotherapy was based on patients’ tolerance to the drugs, and follow-ups were performed with a long duration (two years).
The clinical manifestations were followed up in each therapeutic cycle, and the evaluation was performed according to the routine blood tests and hepatic and renal function. At the end of the third and sixth cycle of chemotherapy, magnetic resonance imaging (MRI) was performed respectively and compared with the preoperative and postoperative imaging data in order to determine the changes of the tumor entities.
Results
The ages of the enrolled patients ranged from 18–55 years old. Among these patients, 30 patients had astrocytomas (grade III), while 37 patients had ependymomas (grade III). In the experimental group, 30 patients with negative MGMT expression and gene promoter methylation were treated with TMZ; 17 patients with positive MGMT protein expression or without methylation were treated with TMZ plus DDP. The baseline characteristics of these patients are described in Table 1.
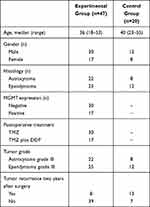 |
Table 1 The Patient Characteristics |
In the experimental chemotherapy group, 39/47 patients had no tumor recurrence, while eight patients had tumor recurrence. The response rate of therapy was 82.9%. Two years after surgery, 37 patients had improved symptoms, and two patients had the same symptoms as before surgery, while symptoms deteriorated in eight cases. The side effects and bone marrow toxicity during chemotherapy were all mild.
For the 20 patients in the control group, no apparent increase in tumor size was observed in seven cases, while an increase in tumor size was observed in 13 cases, and the therapy response rate was 35.0%. Two years after surgery, five patients had improved symptoms, while two patients had the same symptoms as before surgery, and symptoms deteriorated in 13 patients.
The response rate of therapy was significantly higher in the experimental group when compared with the control group.
By comparing the therapeutic effect (recurrence of tumors) between the experimental and control groups, the chi-square test results revealed that P = 0.006, which was <0.01, and means that there was a significant difference. Thus, the therapeutic effect for patients in the experimental group was significantly higher when compared with the control group.
The common adverse effects after drug therapy were nausea, vomiting, and fatigue, while most patients could tolerate the treatment. The side effects of DPP were relatively serious, especially in females during chemotherapy. Tropisetron and mannitol were administered in advance, and no apparent renal dysfunction was found, while elevated transaminase levels in two patients and a transient decrease in white blood cells to 2–3 × 109/L in four patients were observed.
Discussion
The surgical principle of spinal glioma is to resect the tumors as far as possible while preserving the integrity of nerve function.2–25 It has been reported in the literature that spinal cord radiotherapy may increase the risk of spinal cord atrophy, local tissue edema, and even cause paraplegia.2 Furthermore, glioplasia at the site of radiotherapy makes it difficult to determine the resection boundary, thereby making it challenging to resect tumors during the second surgery.3 In clinical practice, performing chemotherapy in patients with primary spinal glioma after surgery relies on the operator’s experience in intracranial glioma chemotherapy.4
Gliomas are the most common tumors of the central nervous system, accounting for 35.26%~60.96% (44.69% on average) of intracranial tumors, and are one of the primary tumors that cause death. Current studies suggest that MGMT plays a vital role in tumor cells resistant to alkylating agents (hypobuluids, TMZ, etc.), and gene mutation is not only an indicator of early tumor formation of gliomas but also a signal of malignant progression of tumors. MGMT is an indicator of drug resistance to alkylating agents in neurogliomas, and this effect has been confirmed by researchers.5 MGMT is a specific DNA repair enzyme that can repair DNA damage caused by alkylation, and the absence of MGMT can increase the incidence of cancer and be used as an omen of early cancer.6 It is suggested that the O6-methylguanine formed by the interaction of TMZ and cellular DNA could be repaired by MGMT, thereby depleting MGMT and play an essential role in overcoming drug resistance.6 Thus, it has been regarded that MGMT might play an important role in tumorigenesis and drug resistance.7 MGMT is a DNA-repair enzyme known to reverse DNA damage caused by alkylating agents such as TMZ, leading to a tumor’s resistance to chemotherapy such as TMZ and nitrosourea.7 MGMT promoter methylation silences suppressed MGMT activity, thus making tumor cells more sensitive to alkylating agent therapy.7 MGMT promoter methylation is especially useful as a treatment option for older patients with high-grade (grades III–IV) gliomas. MGMT promoter methylation was significantly associated with IDH mutations and genome-wide epigenetic changes.7 MGMT promoter methylation confers a survival advantage to glioblastoma and is used in stratified risk studies in clinical trials. MGMT promoter methylation is especially useful as a treatment option for older patients with high-grade (grades III–IV) gliomas. Patients with glioblastoma without methylation benefited less from TMZ chemotherapy than those with MGMT, the methylated promoter.7
In order to reduce the range of laminotomy, a preoperative vertebral positioning examination is not negligible.3 Preoperative localization is usually performed by X-ray or computed tomography (CT). Before cutting through the endorachis, when the tension of the dura mater is high, mannitol can be intravenously infused to reduce the pressure and swelling of the spinal cord, allowing the arrangement of the operation to be clearly defined.8 Experiments have proven that this can significantly reduce infection and cerebrospinal fluid leakage after the operation.2–17 For unilateral intramedullary tumors, incisions can be made along the weakest border between the tumors and the spinal cord to reduce unnecessary injury.9 Electrophysiological monitoring is routinely performed during the surgery.13,21,26,27 When separating tumors, the incision should be strictly performed following the boundary between the spinal cord and tumors.21 Blood vessels should be protected to prevent spinal cord ischemia after surgery.3–15 In case there is close adhesion between the spinal cord and tumors, the boundary of the tumors is unclear, or the changes in electrophysiological monitoring are obvious, the tumors should not be forcibly resected, in order not to injure the normal spinal cord by mistake and cause irreversible neurological impairment.2–7
For patients with multistage tumors or bone destruction, the postoperative stability of the spine should be considered.13 All patients underwent one-stage posterior pedicle screw fixation while resecting the tumors and decompressing the vertebral lamina.13,26 Sridhar et al2 considered that extensive laminectomy during surgery would affect the stability of the spine, and internal fixation and bone graft fusion were needed. When the present team operated on patients with more than two segments of spinal gliomas (Figure 3), all posterior column structures of the spine were destroyed, and iatrogenic spinal instability was caused by total laminectomy. Therefore, appropriate internal fixation and intertransverse process bone graft fusion were selected to ensure the stability of the corresponding segment of the spinal cord.13,26
The postoperative treatment of spinal glioma is a complex problem for neurosurgery, and the therapeutic effect remains unsatisfactory.22 Most researchers advocate comprehensive treatment, which includes surgical resection of the tumors, supplemented by chemotherapy and radiotherapy.23 At present, surgical resection has been achieved at a high level, but controversy exists regarding postoperative radiotherapy. Even the minor injury caused by postoperative radiotherapy for spinal glioma may lead to severe complications, such as paraplegia.22,23 Radiation myelopathy is the most distressing problem for cancer radiotherapists, which may cause vascular occlusion, spinal cord edema, demyelination of the white matter, and even spinal cord necrosis, resulting in paraplegia, cauda equina syndrome, and so on.3–15,27,28
Furthermore, there is a lack of specific therapy for radiation myelopathy.27 It has been reported that neuronutrition, vasodilation, hyperbaric oxygen, and high dose vitamins are not effective during treatment, while high dose corticosteroids can alleviate symptoms in some patients.3–11,21–24,27,28 At present, radiotherapy is generally used as an adjuvant treatment for incompletely resected spinal gliomas in China.28 However, in recent years, based on clinical observations, some researchers consider that radiation therapy can aggravate spinal cord injury and subsequently cause serious spinal cord complications. The scarring of the lesions after radiation therapy may affect the second surgery, which increases the difficulty of the operation.3,27 Although the cell composition of the spinal cord and brain is the same, the incidence of spinal glioma is lower than that of brain glioma.27 The postoperative chemotherapy of brain glioma has matured in China and abroad, but the potential to investigate further has been minimal.27 The investigators attempted to perform chemotherapy for spinal glioma, and the results were satisfactory. As far as surgery is concerned, microsurgery can only remove the local tumors that can be detected under a microscope.27 However, removing all local invasive lesions would be impossible, inevitably leaving a hidden risk of recurrence. In the case of radiotherapy, the individual can only accept a limited dose of radiation, and it is impossible to repeatedly irradiate or carry out total body irradiation.27 Therefore, exploring a low toxicity anti-glioma drug is urgently needed for the present treatment of this disease. TMZ has been revealed to have a good antineoplastic effect in malignant glioma by many clinical studies from China and abroad.13,22–24,26,28–31 Regarding the present medical treatment, based on the microsurgical resection of tumors, it is necessary to give antineoplastic drugs to kill or inhibit the remaining tumors after surgery, improving the survival rate of patients with glioma and reducing the recurrence rate.
In summary, microsurgery for spinal gliomas is rapidly developing in neurosurgery. Since spinal gliomas are malignant tumors, there will be a recurrence of tumors after surgery. Due to the particularity of the spinal cord, the formation of a scar on the spinal cord after radiotherapy affects the second surgery. Furthermore, the follow-up treatment of microsurgery for spinal glioma remains a blank in neurosurgery. The present study provides a new method for the postoperative treatment of spinal glioma.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki.This study was conducted with approval from the Ethics Committee of Beijing Sanbo Brain Hospital Affiliated to Capital Medical University.A written informed consent was obtained from all participants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gramatzki D, Roth P, Felsberg J, et al. Chemotherapy for spinal gliomas in adults: a retrospective study. Neuro Oncol. 2018;20(Suppl 6):vi240. doi:10.1093/neuonc/noy148.993
2. Fujiwara Y, Manabe H, Izumi B, et al. Remarkable efficacy of temozolomide for relapsed spinal myxopapillary ependymoma with multiple recurrence and cerebrospinal dissemination: a case report and literature review. Eur Spine J. 2018;27(Suppl 3):421–425. doi:10.1007/s00586-017-5413-z
3. Chaskis E, Minichini V, Luce S, et al. [Contribution of temozolomide chemotherapy for intramedullary grade II spinal cord astrocytomas in adults: our experience]. Neurochirurgie. 2017;63(4):297–301. Romanian. doi:10.1016/j.neuchi.2017.05.002
4. Velz J, Neidert MC, Struckmann K, et al. A rare case of diffuse midline glioma, H3 K27M mutant, of the spinal cord mimicking meningitis. SN Comprehen Clin Med. 2019;1(1):15–19. doi:10.1007/s42399-018-0007-6
5. Teng YD, Abd-El-Barr M, Wang L, et al. Spinal cord astrocytomas: progresses in experimental and clinical investigations for developing recovery neurobiology-based novel therapies. Exp Neurol. 2019;311:135–147. doi:10.1016/j.expneurol.2018.09.010
6. Prelaj A, Rebuzzi SE, Caffarena G, et al. Therapeutic approach in glioblastoma multiforme with primitive neuroectodermal tumor components: case report and review of the literature. Oncol Lett. 2018;15(5):6641–6647.
7. Wei J, Yang G, Hao X, et al. A multi-sequence and habitat-based MRI radiomics signature for preoperative prediction of MGMT promoter methylation in astrocytomas with prognostic implication. Eur Radiol. 2018;29(2):877–888.
8. Choi SH, Yoon HI, Yi S, et al. Treatment outcomes of radiotherapy for primary spinal cord glioma. Strahlenther Onkol. 2019;195(2):164–174. doi:10.1007/s00066-018-1366-3
9. Diaz-Aguilar D, ReFaey K, Clifton W, et al. Prognostic factors and survival in low grade gliomas of the spinal cord: a population-based analysis from 2006 to 2012. J Clin Neurosci. 2019;61:14–21. doi:10.1016/j.jocn.2018.11.025
10. Rovin RA, Winn R. Expression of O6-methylguanine-deoxyribose nucleic acid methyltransferase and temozolomide response in a patient with a malignant spinal cord astrocytoma. Case report. J Neurosurg Spine. 2007;6(5):447–450. doi:10.3171/spi.2007.6.5.447
11. Tseng HM, Kuo LT, Lien HC, et al. Prolonged survival of a patient with cervical intramedullary glioblastoma multiforme treated with total resection, radiation therapy, and temozolomide. Anticancer Drugs. 2010;21(10):963–967. doi:10.1097/CAD.0b013e32833f2a09
12. Wang GH, Yang J, Wang ZC. Surgical treatment strategy and efficacy of intramedullary ependymoma of spinal cord. Chin J Minimal Invasive Neurosurg. 2010;15(3):99–101.
13. Kim WH, Yoon SH, Kim CY, et al. Temozolomide for malignant primary spinal cord glioma: an experience of six cases and a literature review. J Neurooncol. 2011;101(2):247–254. doi:10.1007/s11060-010-0249-y
14. Raco A, Piccirilli M, Land A, et al. High-grade intramedullary astrocytomas: 30 years experience at the Neurosurgery Department of the University of Rome“Sapienza”. J Neurosurg Spine. 2010;12:144–153. doi:10.3171/2009.6.SPINE08910
15. Kaley TJ, Mondesire-Crump I, Gavrilovic IT. Temozolomide or bevacizumab for spinal cord high-grade gliomas. J Neurooncol. 2012;109(2):385–389. doi:10.1007/s11060-012-0905-5
16. Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87(2):173–179. doi:10.1007/s11060-007-9507-z
17. Eroes CA, Zausinger S, Kreth FW, et al. Intramedullary low grade astrocytoma and ependymoma.Surgical results and predicting factors for clinical outcome. Acta Neurochir. 2010;152:611–618. doi:10.1007/s00701-009-0577-x
18. Benes V, Barsa P, Benes V, Suchomel P. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J. 2009;18(10):1397–1422. doi:10.1007/s00586-009-1076-8
19. Chamberlain MC. Temozolomide for recurrent low-grade spinal cord gliomas in adults. Cancer. 2008;113(8):1019–1024. doi:10.1002/cncr.23677
20. Chamoun RB, Alaraj AM, Al Kutoubi AO, et al. Role of temozolomide in spinal cord low grade astrocytomas: results in two paediatric patients. Acta Neurochir (Wien). 2006;148(2):175–179. doi:10.1007/s00701-005-0694-0
21. Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. doi:10.1227/01.NEU.0000215948.97195.58
22. Nehru GA, Pai R, Samuel P, et al. Status of O6-methylguanine-DNA methyltransferase [MGMT] gene promoter methylation among patients with glioblastomas from India. Neurol India. 2012;60(5):481–486. doi:10.4103/0028-3886.103190
23. Christians A, Hartmann C, Benner A, et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS One. 2001;52(1):85–94.
24. Kim MS, Chung CK, Choe G, et al. Intramedullary spinal cord astrocytoma in adults: postoperative outcome. J Neurooncol. 2001;52(1):85–94. doi:10.1023/A:1010680924975
25. Senhaji Mouhri Z, Goodfellow E, Jean-Claude B. A type I combi-targeting approach for the design of molecules with enhanced potency against BRCA1/2 mutant- and O6-methylguanine-DNA methyltransferase (mgmt)- expressing tumour cells. BMC Cancer. 2017;17(1):540. doi:10.1186/s12885-017-3504-1
26. Chang UK, Choe WJ, Chung SK, et al. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57(2):133–139. doi:10.1023/A:1015789009058
27. Yu LS, Yu HX, Qu BH, Zhang DY. MR diagnosis and clinical stage observation of 55 cases of radioactive myelopathy. Chin J Pract Nervous Dis. 2009;12(14):49–50.
28. Xu XT, Li L, Zhou JY, et al. Retrospective analysis for prognostic factors of primary spinal cord glioma treated with radiation therapy. Tumour. 2005;25(3):257–259.
29. Shen D, Yang QY, Chen ZP. Research progress of temozolomide in malignant glioma chemotherapy. Chin J Neuro Oncol. 2010;8(4):271–276.
30. Xu HS, Zhang JY, Yue WY, et al. Relationship between MGMT expression in gliomas and in vitro their sensitivity to drug as well as its clinical significance. Chin J Clin Neurosurg. 2007;12(5):263–266.
31. Qiu ZK, Feng BH, Chen ZP. Advances in the expression of MGMT in glioma. Chin J Neuro Oncol. 2010;8(1):44–50.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

