Back to Journals » International Journal of General Medicine » Volume 15
A Profile of Nanoparticle-Based Plasma Neurodegenerative Biomarkers for Cognitive Function Among Patients Undergoing Hemodialysis
Authors Chen JB, Chang CC, Moi SH, Li LC
Received 1 April 2022
Accepted for publication 5 July 2022
Published 11 July 2022 Volume 2022:15 Pages 6115—6125
DOI https://doi.org/10.2147/IJGM.S368987
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jin-Bor Chen,1,2 Chiung-Chih Chang,2,3 Sin-Hua Moi,4 Lung-Chih Li1,2
1Division of Nephrology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and School of Medicine, Kaohsiung, 833, Taiwan, Republic of China; 2College of Medicine, Chang Gung University, Taoyuan, 330, Taiwan, Republic of China; 3Department of Neurology, Kaohsiung Chang Gung Memorial Hospital and School of Medicine, Kaohsiung, 833, Taiwan, Republic of China; 4Center of Cancer Program Development, E-Da Cancer Hospital, I-Shou University, Kaohsiung, 833, Taiwan, Republic of China
Correspondence: Jin-Bor Chen, Division of Nephrology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, 123 DaPei Road, Niao Song District, Kaohsiung, 833, Taiwan, Republic of China, Tel +886-7-7317123, ext 8306, Fax +886-7-7322402, Email [email protected]; [email protected]
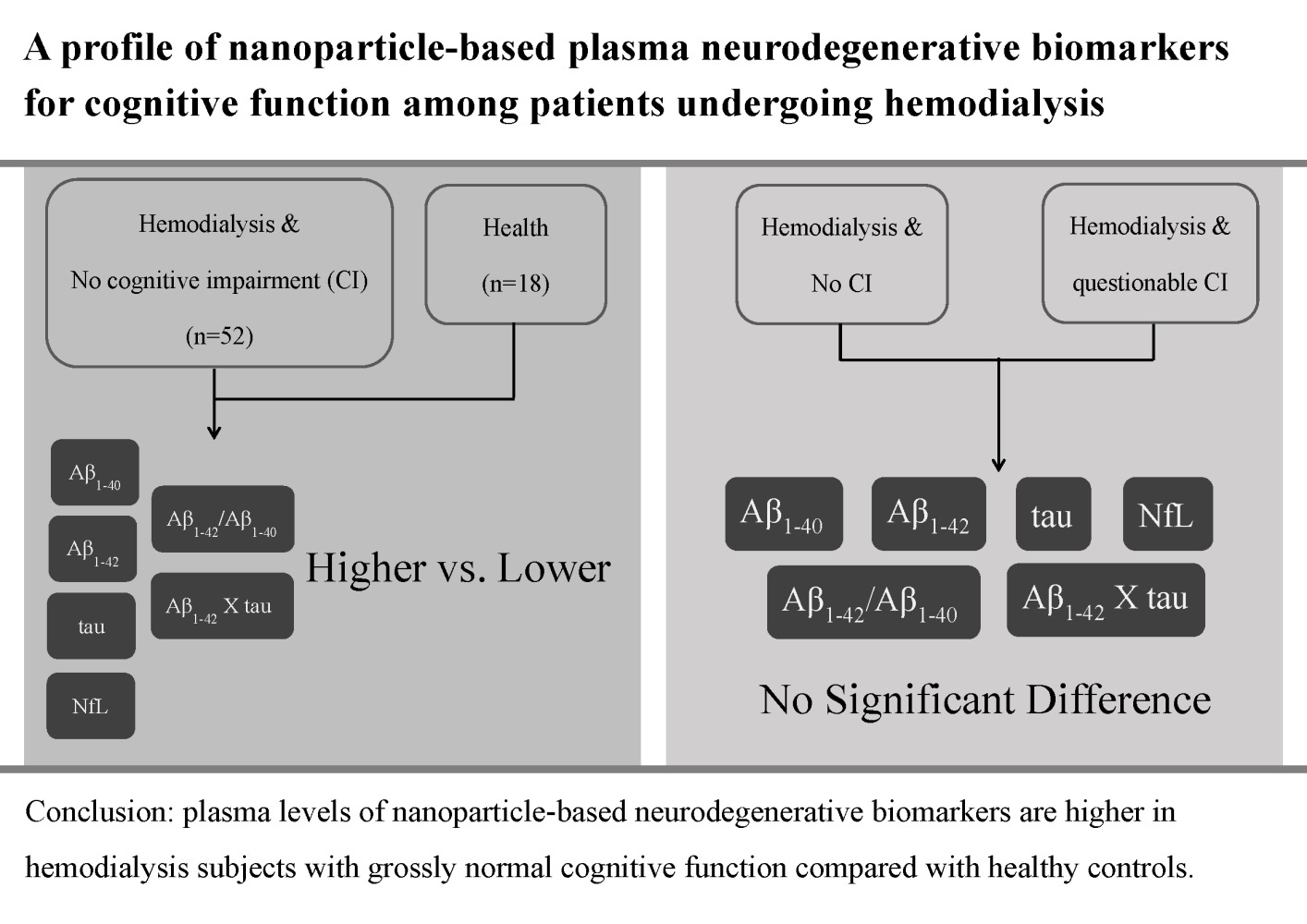
Purpose: This study aimed to compare the plasma levels of nanoparticle-based neurodegenerative biomarkers between hemodialysis (HD) participants with grossly normal cognitive function and healthy controls.
Patients and Methods: A cohort of participants undergoing maintenance HD and healthy controls were enrolled for comparison between July and October 2021. The immunomagnetic reduction method was used to measure plasma neurodegenerative biomarkers Aβ1-40, Aβ1-42, tau protein, and neurofilament light chain (NfL). The clinical dementia rating (CDR) was used to evaluate cognitive function. A receiver operating characteristic curve was used to discriminate between HD participants and healthy controls.
Results: There were 52 and 18 participants in the HD and healthy control groups, respectively. The mean age of the HD participants was 62 years, and that of the healthy controls was 57 years. The mean HD vintage in the HD cohort was 11.8 years. HD participants demonstrated significantly higher plasma levels of Aβ1-42, tau protein, Aβ1-42 × tau, and NfL and Aβ1-42/Aβ1-40 ratio and significantly lower plasma Aβ1-40 levels than healthy controls. The measured plasma biomarkers could not discriminate between CDR0 and CDR0.5 HD participants. The area under the curve of the study biomarkers to discriminate HD participants from healthy controls ranged from 0.987 (Aβ1-42 × tau) to 0.889 (NfL).
Conclusion: The plasma levels of nanoparticle-based neurodegenerative biomarkers were higher in HD participants with grossly normal cognitive function than in healthy controls. These findings imply that neurodegenerative changes appear in HD participants. A profile of plasma neurodegenerative biomarkers could be considered a potential surrogate for evaluating long-term cognitive function in HD participants.
Keywords: Aβ proteins, cognitive function, hemodialysis, tau protein
Graphical Abstract:
Introduction
Patients with chronic kidney disease (CKD) and those undergoing dialysis are at risk of cognitive impairment (CI).1,2 The clinical characteristics of CI include language and memory deficits, cognitive slowing, and diminished executive function. The potential pathological factors include white matter disease,3 silent brain infarcts,4 demographic profiles,5 vascular lesions,6 uremic toxin retention,7 uremic hyperparathyroidism,8 and hemodialysis (HD)-related disequilibrium brain insult.9 CI in CKD and dialysis patients leads to disability in ordinary life, increasing the incidence of hospitalization and mortality.10 Based on our previous study2 and the aforementioned studies, uremic milieu contributes to degenerative changes in the brain. Reports have demonstrated that aberrant functional connectivity in the default mode network exists in the medial frontal lobe, parietal lobe, and thalamus in dialysis patients.11,12 Additionally, the ventral striatum volume is correlated with memory, execution, and depression in dialysis patients.13 Conclusively, CKD and dialysis populations often present with neuropsychological impairments that are correlated with alterations in the neurohormone levels and default in the nervous network.
The clinical characteristics of CI in CKD resemble those observed in Alzheimer’s disease (AD) and parkinsonism. The main pathological hallmarks of AD are the presence of a large number of senile plaques and neurofibrillary tangles in the cerebral cortex and hippocampus.14,15 The major component of senile plaques in AD is the Aβ protein. In patients with CKD, the serum Aβ protein levels are higher than usual, resulting from decreased clearance of Aβ proteins in the blood.16 This observation suggests that AD and CKD share similar pathological processes. Another pathological change in AD is the presence of neurofibrillary tangles. Abnormal phosphorylated tau proteins are the main constituent proteins of neurofibrillary tangles.17 Previous study in aluminum overdose in dialysis patients has shown that association between changes of tau proteins and AD-like brain pathological changes.18 Given the aforementioned observations, CKD and AD share potential markers for CI, such as increased Aβ protein and tau protein expression. Therefore, measuring these markers in the blood may be used as a proxy for disease progression.
Blood-based marker detection has been reported to differentiate between healthy individuals and those with mild CI due to AD or AD-like dementia.15,19,20 However, the levels of these markers in the blood are extremely low, commonly at the level of pg/mL. Recently, ultrasensitive assay techniques have been developed to substantially increase the sensitivity and reliability. These assays include modified enzyme-linked immunosorbent assay, surface-based fluorescence intensity distribution, multiplex electrochemiluminescence, multiplexed flow-matrix analysis, selected reaction monitoring mass spectrometry, single-molecule array, and immunomagnetic reduction (IMR).21,22 Several previous studies have shown the feasibility of the IMR methods for detecting nanoparticles such as Aβ and tau proteins in AD and CI populations.21,23 Accordingly, disease progression can be followed up, and a novel therapeutic strategy could be expected.
We hypothesized that potential nanoparticle-based plasma neurodegenerative biomarkers could be detected using the IMR method in HD participants with grossly normal cognitive function. Consequently, a monitoring protocol can be developed to reduce the incidence of CI in HD participants.
Materials and Methods
Participants
The study was conducted from July to October 2021. Patients who received maintenance outpatient HD thrice weekly at Kaohsiung Chang Gung Memorial Hospital in Taiwan were enrolled. The inclusion and exclusion criteria were followed up in our previous study.2 The inclusion criteria were age of ≥18 years and the ability to provide basic interviews. The exclusion criteria included history of cerebral stroke or brain injury by nonmedical causes; drug-usage history, which may influence cognitive function, including chemotherapy; other comorbidities, which may affect cerebral function, for examples, liver cirrhosis, cancer, and psychiatric diseases; malnutrition, defined by a serum albumin level of <3.5 g/dL; chronic alcohol use; pregnancy; hospitalization within 3 months prior to enrollment; and absence of caregiver for providing medical history.2 Dialyzers for HD had a surface area of 2.0 m2 and used bicarbonate-based dialysate. A total of 100 eligible HD patients were screened. Finally, 52 HD participants were enrolled in the analysis (Figure 1). Healthy controls were recruited voluntarily in the outpatient clinic by posting protocol notifications. A total of 18 healthy controls were enrolled because of a shortage of research budget and time restriction in the poster declaration.
 |
Figure 1 Participants’ deposition. |
Data Collection and Analytic Parameters
Informative data included demographics, educational levels, comorbidities, HD vintage, and circulating parameters. All blood samples from HD participants were obtained mid-week (Wednesday and Thursday). The measured parameters included serum albumin, urea nitrogen, creatinine, calcium, phosphate, cholesterol, triglyceride, ferritin, intact parathyroid hormone, hemoglobin, HD adequacy index (Kt/V), urea (Daugirdas method),24 Aβ1-40, Aβ1-42, tau protein, and neurofilament light chain (NfL). Blood samples for biochemical measurements were obtained using commercial kits and an autoanalyzer (Hitachi 7600–210, Hitachi Ltd., Tokyo, Japan). The albumin levels were measured by the bromocresol green method. Blood drawn from participants was collected in ethylenediaminetetraacetic acid (EDTA) collection tubes, centrifuged according to the instructions of the package inserts, and stored at −80 °C until use in plasma Aβ1-40, Aβ1-42, tau protein, and NfL biomarker assays. Finally, blood samples in a dry-ice package were delivered to MagQu Co., Ltd., in New Taipei City, Taiwan, for biomarker measurements. The IMR method used to detect plasma Aβ1-40, Aβ1-42, tau protein, and NfL followed the protocol described in a previous study.25
Mini-Mental State Examination
General cognitive function was assessed using the Mini-Mental State Examination (MMSE), which has been validated in a Chinese population.26 We used clinical dementia rating (CDR) scores to stratify the functional scores of HD participants.2,27 A rating score of CDR0 indicated no dementia, and CDR0.5, CDR1, CDR2, and CDR3 indicated questionable, mild, moderate, and severe dementia, respectively. The necessary information was obtained through interviews with the patients and reliable informants (eg, family members) by a qualified reviewer based on the CDR assessment protocol.
Statistical Analysis
The baseline demographic characteristics and laboratory measurements of HD participants and healthy controls are presented as frequency (percentage), mean (standard deviation), and median (interquartile range). The difference in distribution between subgroups was estimated using the chi-square test, Fisher exact test, independent two-sample t-test, or Wilcoxon rank-sum test. The Pearson and Spearman correlation tests were conducted to evaluate the correlation between the CI plasma biomarkers and associated clinical factors. Receiver operating characteristic (ROC) curve analysis was performed to discriminate between HD participants and healthy controls according to CI plasma biomarkers. Statistical significance was set at p<0.05. All analyses were performed using R software (R Development Core Team 2021, version 4.0.5).
Results
Baseline Characteristics
The mean age of the HD participants was 62 years, and that of the healthy controls was 57 years. There were equal numbers of men and women in the HD and healthy control groups. HD participants had comorbidities, diabetes (n=15), hypertension (n=44), and cardiovascular disease (n=15). Most of the participants reached the 12-year educational level: the HD participants (n=40) and healthy controls (n=16). HD participants demonstrated significantly higher plasma levels of Aβ1-42, tau protein, Aβ1-42 × tau, and NfL and a greater Aβ1-42/Aβ1-40 ratio than healthy controls. In contrast, the Aβ1-40 levels were significantly lower in the HD group than in healthy controls (Table 1).
 |
Table 1 Baseline Characteristics |
Demographics and Plasma Biomarkers in HD Patients
Table 2 presents the dichotomous results for various demographic and laboratory factors between CDR0 and CDR0.5 in HD participants (n=9). Patients in CDR0.5 showed significantly lower serum albumin and triglyceride levels than those in CDR0. The levels of Aβ1-42, Aβ1-40, tau protein, NfL, and Aβ1-42 × tau and Aβ1-42/Aβ1-40 ratio were not significantly different between the CDR0 and CDR0.5 groups.
 |
Table 2 Demographics and Plasma Biomarkers in Hemodialysis Patients (n=9) |
Correlation Analysis Results for Plasma Biomarkers in HD Participants
In the correlation analysis, the plasma tau protein levels were significantly negatively associated with sex and Kt/V-urea. The plasma Aβ1-40 levels were also negatively associated with hypertension. The plasma Aβ1-42 levels were significantly positively associated with cholesterol levels (Table 3).
 |
Table 3 Correlation Analysis Results for Plasma Biomarkers in Hemodialysis Subjects (n=52) |
ROC Curve Analysis for Discriminating HD Participants from Controls
Table 4 shows the ROC curve analysis of plasma biomarkers for discriminating HD participants from healthy controls. The area under the curve (AUC) for all plasma biomarkers was >0.9, except for NfL (0.889). The distribution of sensitivity in all plasma biomarkers ranged from 0.750 (Aβ1-40) to 0.962 (Aβ1-42 × tau). The specificity distribution ranged from 0.889 (tau and NfL) to 0.962 (Aβ1-42 × tau). Figure 2 shows the ROC curve analysis using figure expression.
 |
Table 4 ROC Analysis for Discriminating Hemodialysis Subjects from Controls |
 |
Figure 2 Receiver operating characteristic curve analysis for discriminating hemodialysis participants from controls. |
Spearman Correlation Between MMSE Parameters in HD Participants
Table 5 shows correlation estimation between MMSE parameters (orientation to time, place, attention and calculation, recall, and language) and plasma biomarkers in HD participants (n=7). There was no significant correlation between them.
 |
Table 5 Spearman Correlation Between Orientation to Time, Orientation to Place, Attention and Calculation, Recall, Language and Plasma Biomarkers in Hemodialysis Subjects (n = 7) |
Discussion
The end-stage renal disease population has been recognized to have a higher incidence of CI than the general population.28,29 Accordingly, there is an urgent need to determine a practical and validated method for early detection of CI in end-stage renal disease populations. Furthermore, it is more complicated in the HD population owing to the complex uremic milieu and solute clearance during HD sessions. Based on previous reports, there are similar clinical characteristics of CI between CKD, AD, and parkinsonism.14,15,18,30 Therefore, detection of plasma Aβ proteins is considered a reasonable method for screening for CI in end-stage renal disease populations. Moreover, the plasma Aβ protein levels increase when kidney function is reduced.31 Consequently, it is reasonable to expect the plasma Aβ protein levels to increase in HD participants compared with those in healthy controls. However, it is unclear whether increased plasma Aβ protein levels are related to cognitive function in the HD population. Moreover, the relationships between other plasma nanoparticle-based neurodegenerative biomarkers, such as tau and NfL, and cognitive function in the HD population also need to be further investigated. In the present study, we compared nanoparticle-based plasma neurodegenerative biomarkers between HD participants with normal cognitive function and healthy controls. We found higher plasma levels of Aβ1-42, tau protein, Aβ1-42 × tau, and NfL and a greater Aβ1-42/Aβ1-40 ratio in HD participants than in healthy controls (Table 1). Moreover, there was a high AUC value for discriminating HD participants from healthy controls (Table 4). Further research is required to establish whether long-term blood monitoring of neurodegenerative components plays a role in reducing the incidence of CI in HD participants.
HD can affect the plasma levels of Aβ1-40, Aβ1-42, and tau protein. In a previous study, hemodialyzers effectively removed blood Aβ1-40 and Aβ1-42 during HD sessions; in contrast, hemodialyzers could not remove tau protein.32 However, tau protein was observed to have a small influx into blood during HD sessions.32 One speculation is that the efflux ability of the blood–brain barrier may be disturbed by uremic toxins, especially during HD sessions,33 thus resulting in a small increase in the blood tau protein levels. In addition, the plasma t-tau levels were reported not to be dependent on HD vintage in a cross-sectional study.32 On the contrary, the plasma Aβ1-40 and Aβ1-42 levels significantly decline in longer HD vintage, especially in cases exceeding 4 years.32 However, these results in clinical observational studies need to be further validated by well-designed studies in the future. Our HD participants had received HD therapy for more than 4 years. Based on previous clinical observations, we assumed that the plasma levels of Aβ1-40, Aβ1-42, and tau protein in our HD participants were relatively constant in cross-sectional measurements and could be applied to examine their associations with cognitive function in our HD cohort.
Higher plasma levels of Aβ1-42 and a greater Aβ1-42/Aβ1-40 ratio have been reported in healthy controls that do not transition to cognitive decline.34 It has also been observed that an increased Aβ1-42/Aβ1-40 ratio and Aβ removal via HD are related to slight cognitive function improvement or maintenance of cognitive function in HD participants.35 In the present study, HD participants demonstrated significantly higher plasma levels of Aβ1-42 and a greater Aβ1-42/Aβ1-40 ratio than healthy controls. The peripheral Aβ levels are affected by age and gene expression: increased Aβ efflux in the blood–brain barrier at the choroid plexus with transporter genes LRP-1 and P-gp and decreased Aβ influx with transporter gene LRP-2.36 Thus, increasing age demonstrated more Aβ retention in the cerebrospinal fluid.36 Moreover, aberrations in gene expression (single nucleotide polymorphisms, DNA methylation, miRNA, and lincRNA) could decrease the Aβ levels in the blood.34 We did not examine gene expression in our participants. Therefore, we hypothesized that this limitation may contribute to these contradictory results. Further comparison between CDR0 and CDR0.5 HD participants revealed no significant differences in the plasma levels of Aβ1-42 or Aβ1-42/Aβ1-40 ratio between the two groups (Table 2). Due to the limited cross-sectional study and small number of HD participants, we could not ascertain whether the higher plasma levels of Aβ1-42 and greater Aβ1-42/Aβ1-40 ratio observed in our HD participants would result in favorable cognitive function, given a longer observational duration. A similar uncertain situation also exists in discriminating between normal cognitive function and subtle cognitive function, CDR0 from CDR0.5, in HD participants using plasma Aβ measurements.
NfL is a component of neuronal axon cytoskeleton. HD participants are vulnerable to axon damage through several mechanisms. These pathogenic mechanisms include uremic toxins, reduced cerebral blood perfusion during an HD session, sympathetic hyperactivation, and diabetes.37 Hou et al reported that the plasma NfL levels were marginally higher in HD participants with CI than in those without CI.37 In our study, HD participants were considered to have normal cognitive function or subtle CI based on the CDR values. Thus, we did not find a significant difference in the plasma NfL levels between the two groups (Table 2). However, we are aware that a small number of participants may bias this result, and our results await further study.
An association between anemia and hypoalbuminemia with CI in adults has been reported.38,39 A correlation has also been reported between HD participants with CI and lower serum albumin and hemoglobin levels.37 In addition, the hemoglobin level was negatively correlated with striatum function in HD participants.38 Our HD participants were well nourished and presented with normal hemoglobin levels based on laboratory measurements. Thus, we suppose that this is one of the reasons why it was not possible to distinguish between normal and subtle CI in our HD participants. Through correlation analysis, we found that plasma tau protein was weakly negatively correlated with sex and Kt/V-urea and Aβ1-40 were weakly negatively correlated with hypertension. In contrast, the Aβ1-42 level was positively correlated with cholesterol levels (Table 3). In a healthy Taiwanese population study, men had more comorbidities than women.40 Our HD participants had similar comorbidities and Kt/V-urea values in men and women (data not shown). Therefore, an exact explanation for the results of the correlation analysis cannot be drawn from our study. A larger sample size and longer observational period are necessary to address this epiphenomenon in the HD population.
Our study has a few limitations. First, we measured plasma biomarkers for cognitive function at one time point. Considering the time effect on cognitive function changes, a longitudinal observation is optimal for evaluating the patterns of these biomarkers between HD participants and healthy controls. Second, it is difficult to determine whether a participant is cognitively normal. Mental tests, such as the MMSE, are time-consuming and rely heavily on individual understanding. Therefore, it is reasonable to evaluate cognitive function using sufficient longitudinal data or consensus tests. Our HD participants were considered to have normal or subtle CI, based on their MMSE test and CDR values. Concerning the difficulty of completing the MMSE test in participants with advanced CI, underestimation cannot be completely ruled out in our study. However, it is worth investigating the clinical significance of nanoparticle-based plasma neurodegenerative biomarkers in HD participants with advanced CI to predict future outcomes. Third, the number of participants in our study was relatively small, and the study was conducted at one HD center. The study results may not be generalizable to other HD cohorts in individual HD centers. Our study also presents several hotspots for estimating CI using nanoparticle-based plasma neurodegenerative biomarkers in HD participants with grossly normal cognitive function. First, the levels of these plasma biomarkers were higher in HD participants with grossly normal cognitive function than in healthy controls, and they demonstrated a high AUC to distinguish them from healthy controls. These observations provide a possible strategy for using the plasma Aβ, tau, and NfL levels to monitor long-term cognitive function in the HD population. Second, it was difficult to distinguish normal cognitive function from subtle CI in HD participants using the plasma biomarkers tested in our study. Given this result, medical providers need to explore more neurodegenerative biomarkers for the early detection of subtle CI in HD participants. Finally, these plasma biomarkers cannot be correlated with MMSE parameters in HD participants with grossly normal cognitive function. At present, the MMSE is appointed as a formal tool by the Taiwanese government to estimate cognitive function in healthy reimbursement. Our study results raise a discussion on whether the application of other mental evaluation tools in HD participants could be linked to plasma neurodegenerative biomarker measurements. Therefore, a potential therapeutic strategy is possible to prevent CI in HD patients by medical providers.
Conclusions
HD participants demonstrated significantly higher plasma levels of Aβ1-42, tau protein, Aβ1-42 × tau, NfL, and Aβ1-42/Aβ1-40 and significantly lower plasma Aβ1-40 levels than healthy controls. However, these plasma biomarkers cannot be used to distinguish between HD participants with normal cognitive function and those with subtle CI. The application of these plasma neurodegenerative biomarkers to evaluate long-term cognitive function in HD participants requires further investigation.
List of Abbreviations
AD, Alzheimer’s disease; AUC, area under the curve; CDR, clinical dementia rating; CI, cognitive impairment; CKD, chronic kidney disease; HD, hemodialysis; IMR, immunomagnetic reduction; MMSE, Mini-Mental State Examination; NfL, neurofilament light chain; ROC, receiver operating characteristic.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Committee on Human Research at Kaohsiung Chang Gung Memorial Hospital in Taiwan (number: 202100284B0). The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors thank Miss Ya-Wen Hsu for her dedicated to blood sample collection and preparation for laboratory analyses.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Kaohsiung Chang Gung Memorial Hospital in Taiwan, grant number CMRPG8L0851.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. 2011;79:14–22. doi:10.1038/ki.2010.336
2. Chen JB, Chang CC, Li LC, et al. Mutual interaction of clinical factors and specific microRNAs to predict mild cognitive impairment in patients receiving hemodialysis. Cells. 2020;9:2303. doi:10.3390/cells9102303
3. Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. 2008;15:123–132. doi:10.1053/j.ackd.2008.01.010
4. Wada M, Nagasawa H, Iseki C, et al. Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci. 2008;272:36–42. doi:10.1016/j.jns.2008.04.029
5. McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol. 2015;10:2181–2189. doi:10.2215/CJN.01960215
6. Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246–255. doi:10.1016/S1474-4422(08)70040-1
7. Watanabe K, Watanabe T, Nakayama M. Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology. 2014;44c:184–193. doi:10.1016/j.neuro.2014.06.014
8. Chou FF, Chen JB, Hsieh KC, Liou CW. Cognitive changes after parathyroidectomy in patients with secondary hyperparathyroidism. Surgery. 2008;143:526–532. doi:10.1016/j.surg.2007.11.019
9. Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial. 2008;21:29–37. doi:10.1111/j.1525-139X.2007.00384.x
10. Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD-A systematic review. Clin J Am Soc Nephrol. 2016;11:1624–1639. doi:10.2215/CJN.13611215
11. Liang X, Wen J, Ni L, et al. Altered pattern of spontaneous brain activity in the patients with end-stage renal disease: a resting-state functional MRI study with regional homogeneity analysis. PLoS One. 2013;8:e71507. doi:10.1371/journal.pone.0071507
12. Zheng G, Wen J, Zhang L, et al. Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functional MR imaging study. Metab Brain Dis. 2014;29:777–786. doi:10.1007/s11011-014-9568-6
13. O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi:10.1126/science.1094285
14. Hansson O, Zetterberg H, Buchhave P, et al. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi:10.1159/000100926
15. Lui JK, Laws SM, Li QX, et al. Plasma amyloid-beta as a biomarker in Alzheimer’s disease: the AIBL study of aging. J Alzheimers Dis. 2010;20:1233–1242. doi:10.3233/JAD-2010-090249
16. Shi Y, Liu Z, Shen Y, Zhu H. A novel perspective linkage between kidney function and Alzheimer’s disease. Front Cell Neurosci. 2018;12:384. doi:10.3389/fncel.2018.00384
17. Brici D, Götz J, Nisbet RM. A novel antibody targeting tau phosphorylated at serine 235 detects neurofibrillary tangles. J Alzheimers Dis. 2018;61:899–905. doi:10.3233/JAD-170610
18. Harrington CR, Wischik CM, McArthur FK, Taylor GA, Edwardson JA, Candy JM. Alzheimer’s-disease-like changes in tau protein processing: association with aluminium accumulation in brains of renal dialysis patients. Lancet. 1994;343:993–997. doi:10.1016/S0140-6736(94)90124-4
19. Chiu MJ, Yang SY, Horng HE, et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci. 2013;4:1530–1536. doi:10.1021/cn400129p
20. Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–1835. doi:10.1212/WNL.0000000000003246
21. Chiu MJ, Chen TF, Hu CJ, et al. Nanoparticle-based immunomagnetic assay of plasma biomarkers for differentiating dementia and prodromal states of Alzheimer’s disease - A cross-validation study. Nanomedicine. 2020;28:102182. doi:10.1016/j.nano.2020.102182
22. Song C, Deng P, Que L. Rapid multiplexed detection of beta-amyloid and total-tau as biomarkers for Alzheimer’s disease in cerebrospinal fluid. Nanomedicine. 2018;14:1845–1852. doi:10.1016/j.nano.2018.05.013
23. Yang SY, Liu HC, Chen WP. Immunomagnetic reduction detects plasma Aβ(1-42) levels as a potential dominant indicator predicting cognitive decline. Neurol Ther. 2020;9:435–442. doi:10.1007/s40120-020-00215-2
24. Daugirdas JT. The post: pre-dialysis plasma urea nitrogen ratio to estimate K.t/V and NPCR: mathematical modeling. Int J Artif Organs. 1989;12:411–419.
25. Yang SY, Chiu MJ, Chen TF, Horng HE. Detection of plasma biomarkers using immunomagnetic reduction: a promising method for the early diagnosis of Alzheimer’s disease. Neurol Ther. 2017;6:37–56. doi:10.1007/s40120-017-0075-7
26. Yu RL, Lee WJ, Li JY, et al. Evaluating mild cognitive dysfunction in patients with parkinson’s disease in clinical practice in Taiwan. Sci Rep. 2020;10:1014. doi:10.1038/s41598-020-58042-2
27. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi:10.1192/bjp.140.6.566
28. Drew DA, Weiner DE, Sarnak MJ. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis. 2019;74:782–790. doi:10.1053/j.ajkd.2019.05.017
29. Chien CW, Lin YC, Huang SK, Chen PE, Tung TH. A population-based study of the association between hemodialysis and cognitive impairment. Asia Pac Psychiatry. 2020;12:e12404. doi:10.1111/appy.12404
30. Tholen S, Schmaderer C, Chmielewski S, et al. Reduction of Amyloid-β plasma levels by hemodialysis: an anti-amyloid treatment strategy? J Alzheimers Dis. 2016;50:791–796. doi:10.3233/JAD-150662
31. Arvanitakis Z, Lucas JA, Younkin LH, Younkin SG, Graff-Radford NR. Serum creatinine levels correlate with plasma amyloid Beta protein. Alzheimer Dis Assoc Disord. 2002;16:187–190. doi:10.1097/00002093-200207000-00009
32. Kitaguchi N, Tatebe H, Sakai K, et al. Influx of tau and amyloid-β proteins into the blood during hemodialysis as a therapeutic extracorporeal blood amyloid-β removal system for Alzheimer’s disease. J Alzheimers Dis. 2019;69:687–707. doi:10.3233/JAD-190087
33. Bobot M, Thomas L, Moyon A, et al. Uremic toxic blood-brain barrier disruption mediated by AhR activation leads to cognitive impairment during experimental renal dysfunction. J Am Soc Nephrol. 2020;31:1509–1521. doi:10.1681/ASN.2019070728
34. Rembach A, Watt AD, Wilson WJ, et al. Plasma amyloid-β levels are significantly associated with a transition toward Alzheimer’s disease as measured by cognitive decline and change in neocortical amyloid burden. J Alzheimers Dis. 2014;40:95–104. doi:10.3233/JAD-131802
35. Kitaguchi N, Hasegawa M, Ito S, et al. A prospective study on blood Aβ levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer’s disease. J Neural Transm. 2015;122:1593–1607. doi:10.1007/s00702-015-1431-3
36. Pascale CL, Miller MC, Chiu C, et al. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. doi:10.1186/2045-8118-8-21
37. Hou YC, Huang CL, Lu CL, et al. The role of plasma neurofilament light protein for assessing cognitive impairment in patients with end-stage renal disease. Front Aging Neurosci. 2021;13:657794. doi:10.3389/fnagi.2021.657794
38. Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology. 2013;81:528–533.
39. Llewellyn DJ, Langa KM, Friedland RP, Lang IA. Serum albumin concentration and cognitive impairment. Curr Alzheimer Res. 2010;7:91–96. doi:10.2174/156720510790274392
40. Hu CJ, Chiu MJ, Pai MC, et al. Assessment of high risk for Alzheimer’s Disease using plasma biomarkers in subjects with normal cognition in Taiwan: a preliminary study. J Alzheimers Dis Rep. 2021;5:761–770. doi:10.3233/ADR-210310
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
