Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
A Probing of the Issue of Detecting IgG, IgG4 and IgA Antibodies to Laminin 332 Epitopes in Mucous Membrane Pemphigoid: A Clinical-Laboratory Experience of a Single Central European University Dermatology Department
Authors Gornowicz-Porowska J, Jałowska M, Seraszek-Jaros A , Bowszyc-Dmochowska M , Kaczmarek E , Dmochowski M
Received 23 January 2022
Accepted for publication 6 April 2022
Published 27 April 2022 Volume 2022:15 Pages 783—790
DOI https://doi.org/10.2147/CCID.S359589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Justyna Gornowicz-Porowska,1 Magdalena Jałowska,2 Agnieszka Seraszek-Jaros,3 Monika Bowszyc-Dmochowska,4 Elżbieta Kaczmarek,3 Marian Dmochowski2
1Department and Division of Practical Cosmetology and Skin Diseases Prophylaxis, Poznan University of Medical Sciences, Poznan, Poland; 2Autoimmune Blistering Dermatoses Section, Department of Dermatology, Poznan University of Medical Sciences, Poznan, Poland; 3Department of Bioinformatics and Computational Biology, Poznan University of Medical Sciences, Poznan, Poland; 4Cutaneous Histopathology and Immunopathology Section, Department of Dermatology, Poznan University of Medical Sciences, Poznan, Poland
Correspondence: Marian Dmochowski, Autoimmune Blistering Dermatoses Section, Department of Dermatology, Poznan University of Medical Sciences, Poznan, Poland, Tel +48 61 8691319, Email [email protected]
Purpose: Mucous membrane pemphigoid (MMP) is a very rare autoimmune bullous disease, affecting predominantly the mucosae and characterized by autoantibodies to the epithelial basement membrane components. Laminin 332 (Ln-332) is one of the most probable antigens with association with malignancy. The laboratory diagnosis of Ln-332-mediated autoimmunity is troublesome. The aim here was to comparatively examine IgG, IgG4, and IgA autoantibodies specific to α 3, β 3 or γ 2 subunits of Ln-322 in MMP patients using the BIOCHIP mosaic-based indirect immunofluorescence technique (IIF).
Patients and Methods: Sera from 15 MMP patients were studied. BIOCHIP mosaic-based Ln-332 IIF, direct immunofluorescence, ELISA tests for anti-BP180/BP20 IgG antibodies and statistical analyses were performed.
Results: Of all the 15 sera examined for IgG4 antibodies, only 1 (6.67%) reacted with the α 3 chain, 0 with the β 3 chain, and 0 with the γ 2 chain. No positive reactivity was seen with the IgG and IgA antibodies. BIOCHIP mosaic-based IIF with Ln-332 showed 100% sensitivity, 8% specificity, 21% positive predictive value, and 100% negative predictive value in relation to the diagnostic gold standard of DIF. The concomitant malignancies were revealed in three cases.
Conclusion: The detection of antibodies to Ln-332 chains is occasional in Polish MMP sufferers. Still, the evaluation of IgG4 antibodies in MMP can reduce the false-negative results.
Keywords: mucous membrane pemphigoid, laminin 332, immunologic test
Introduction
Various autoimmune bullous diseases (AIBDs) frequently involve oral cavity. Mucous membrane pemphigoid (MMP) is one rare type of AIBDs characterized by chronic, inflammatory and subepithelial blistering lesions that predominantly affect mucous membranes followed by scarring.1,2 MMP, also causing serious complications such as blindness, was previously referred to as cicatricial pemphigoid due to scarring features. The clinical course and prognosis of MMP may depend on the specific autoantigen targeted.3
Immunological background of MMP is associated with autoantibodies directed against different antigens located at the epithelial basement membrane,4 involving intracytoplasmic hemidesmosomal protein BP230, the transmembrane hemidesmosomal proteins BP180 and integrin α6β4, and laminin-332 (Ln-332). Autoantibodies against Ln-332 were suggested to be present in approximately 1/3 of the patients.1 Their pathogenicity has been proved by passive transfer in mice and human skin graft models.1,4 It was also reported that MMP patients with anti-Ln-332 have an increased relative risk of malignancy.5,6 However, the restricted number of patients limits the significance of this observation.
Ln-332, formerly known as kalinin, epiligrin, nicein and laminin-5, is a heterotrimer, which is the most important for the skin integrity.3 Beyond the adhesion function, Ln-332 is associated with cell migration, tissue maturation, and wound repair. Ln-332 consists of the laminin chains (subunits) α3 (165 and 145 kDa), β3 (140 kDa) and γ2 (105 kDa).3,4 By analyzing the immune profile of MMP patients, it was reported that the IgG was mostly directed to the α3 subunit (86%), followed by the β2 subunit (46%) and the γ2 subunit (29%).7 Huang et al8 revealed the distinct roles of the three Ln-332 subunits (α3, β3 and γ2) in cell proliferation, migration, invasion and apoptosis.
Due to severe complications, an early and proper recognition of MMP is essential, allows the timely initiation of the effective therapy, and justifies an extensive tumour screening. However, the diagnostics of anti-Ln-332 MMP is complex due to multiple antigens, and clinical resemblance to other forms of pemphigoid. The pathological immunoresponse may be detected by direct and indirect immunofluorescence (DIF and IIF, respectively), as well as molecular techniques.9 The DIF, a definitive laboratory test for AIBDs, reveals the deposition of IgG autoantibodies and C3, or sometimes of IgA autoantibodies, at the epithelial basement membrane. However, choosing the optimal biopsy site, especially from mucosal tissues, for the DIF to give reliable results often pose difficulties in MMP.10,11 The perilesional tissue obtained can be fragmented or smashed rendering the unequivocal reading impossible. Hence, the serological biochemical-molecular techniques, including salt-split IIF (ssIIF), are diagnostically important. Still, one cannot identify the molecular nature of the target antigen with the ssIIF. Therefore, more technologically advanced serological assays, particularly enabling the detection of less common target antigen are needed. Despite its diagnostic and prognostic significance, immunoassays for the anti-Ln-332 autoantibodies recognition have not yet been established in the clinical laboratory routine.
Previously, it was reported that IgG4 was the most dominant tissue-bound or circulating antibody in bullous pemphigoid (BP) with pathogenic significance. Nevertheless, to the best of our knowledge, there are only scanty data12 regarding the frequency of circulating anti-Ln-332 IgG4 and its importance in MMP. Here, we describe the BIOCHIP mosaic-based IIF modified by us for the determination of not only anti-Ln-332 IgG antibodies but also for IgG4 and IgA antibodies. The BIOCHIP used contained separate α3, β3 or γ2 subunits of Ln-332, so the diagnostic value and frequency of circulating anti-Ln-332 IgG, IgG4 and IgA antibodies to those subunits were comparatively evaluated.
Materials and Methods
The study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the Poznan University of Medical Sciences in Poland (no 540/13). Informed written consent was obtained from each individual. The commercially available transfected HEK293 cells were not separate reagents. They were obtained as an integral part of the IIF BIOCHIP Ln-332 mosaic gifted by Euroimmun, Germany.
Material
Patients
In total, 15 patients with MMP before initiation of treatments advocated for MMP were investigated. The examined group included Slavic patients (7 males and 8 females) at a mean age of 71.5 years (min. 36; max. 90). The inclusion criteria of the patients closely followed the currently accepted diagnostic criteria for MMP,2 and involved critically important clinical signs (presence of mucosal lesions irrespectively of cutaneous lesions), DIF with analysis of serration pattern of deposits of immunoreactants along the epithelial basement membrane as well as IIF, and antigen-specificity analysis of the serum with enzyme-linked immunosorbent assay (ELISA) against BP180 and BP230. This selection of patients showing a diverse range of involvement of various body regions was seen at the Autoimmune Blistering Dermatoses Section, Department of Dermatology, Poznan University of Medical Sciences, Poland, in years 2008–2018. Altogether, three cases (20%) had associated tumour (breast, vulvar, endometrial): two (13.33%) of the 15 MMP patients with clinical data had a malignancy diagnosed before the time of diagnosis of MMP, and one patient was diagnosed with the breast cancer, as an oncological surveillance was introduced, 6 months after MMP diagnosis.
Human Sera
The serum used in the serological tests was obtained at the time of hospital admission/ambulatory care. Five mL of blood serum were obtained from each participant. The samples were centrifuged for 10 min at 3500 rpm. Thereafter, they were stored at –80°C/–20°C until performing ELISA and BIOCHIP mosaic IIF. Mutually control groups for comparison were obtained by evaluating IgG, IgG4, and IgA-mediated immune responses.
Methods
Indirect Immunofluorescence Assay Using Recombinant Laminin 332 (Biochip Mosaic)
The reaction was performed following the manufacturer’s instructions. All sera were subjected to an indirect IF biochip mosaic with six different substrates comprising HEK293 cells transfected with plasmids for i) LAMA3, ii) LAMB3, iii) LAMC2 (encoding for the α3, β3, and γ2 chains, respectively), iv) all three plasmids encoding for the heterotrimer, v) all three plasmids encoding for the heterotrimer and a His-tag, and vi) the empty plasmid (Euroimmun, Germany). All sera were applied in a 1:10 dilution in PBS supplemented with 0.2% Tween20, and after washing, bound autoantibodies were detected by antihuman secondary antibodies. The original BIOCHIP under development by Euroimmun, Germany, for the assessment of anti-Ln-332 IgG was modified by us to enable the assessment of IgG4 and IgA antibodies to those antigens. In the modified IIF, in addition to the fluorescein isothiocyanate (FITC)-conjugated anti-human IgG antibody provided by the BIOCHIP manufacturer, FITC-conjugated anti-human IgA rabbit polyclonal antibody (Dako, Denmark) diluted 1:100, and FITC-conjugated anti-human IgG4 murine monoclonal antibody (Sigma, USA) diluted 1:100 were used. In order to avoid technical errors, each analysis was performed in duplicates by the same operator. The internal positive and negative controls were used.
All the slides were evaluated simultaneously by two observers using a microscope (BX40, Olympus, Japan) with a fluorescent adapter.
Direct Immunofluorescence Procedure
Traditional DIF of perilesional mucosal tissue or, when cutaneous lesions were present, perilesional skin tissue for evaluation of immunoreactants deposition was performed following the procedure previously described.13 The tissue sections were incubated in a humid chamber for 30 minutes at room temperature (RT) with commercially available FITC-conjugated anti-human IgA, IgM, IgG, and C3 rabbit polyclonal antibodies (Dako, Denmark) and FITC-conjugated anti-human IgG subclasses: IgG1 and IgG4 murine monoclonal antibodies (Sigma, USA).
ELISA
The specific circulating serum abs were detected with commercially available ELISAs. ELISAs were performed using the Euroimmun (Luebeck, Germany) ELISA kits: (i) monoanalyte ELISA utilizing recombinant protein BP180, BP230, with the manufacturer’s cut-off value of 20 RU/mL; (ii) multianalyte ELISA containing 6 antigens (DSG1, DSG3, BP180, BP230, envoplakin, type VII collagen), with the cut-off ratio 1. All measurements were made in the ELISA plate reader (Asys Expert 96) equipped with MikroWin 2000 software by a single operator following the manufacturer’s instructions.
Statistical Analysis
The accuracy of mosaic IIF was evaluated by calculating diagnostic sensitivity, diagnostic specificity, as well as positive and negative predictive values in relation to the ELISA using the MedCalc Software 2015 (Ostend, Belgium; www.medcalc.org). Associations in results between tests were assessed using Fisher’s exact test. A p < 0.05 was arbitrarily considered statistically significant.
Results
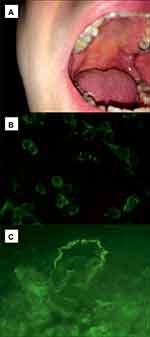
In general, our patients had mainly oral, cutaneous and/or genital mucosal involvement. Only one patient had concomitant conjunctival involvement. The detailed results are shown in Table 1, whereas clinical and microscopic findings in a single patient having anti-α3 chain of Ln-332 IgG4 antibodies are shown in Figure 1.
 |
Table 1 Clinical and Immunological Findings in 15 Patients with Mucous Membrane Pemphigoid |
IgG4 was the sole antibody detected in just 1 young male with MMP. This patient gave no history of malignancy before the diagnosis of MMP, and no malignancy was revealed within the 2-year follow-up period after the diagnosis of MMP. Of all the 15 sera for IgG4, 1 (6.67%) reacted with the α3 chain, 0 with the β3 chain, and 0 with the γ2 chain. No positive reactivity was seen with the IgG and IgA.
The statistical analysis using Fisher’s exact test revealed no statistically significant dependency between anti-Ln-332 IIF and anti-BP180 ELISA, anti-BP230 ELISA, as well as DIF (p > 0.05). The dependencies were not reported between anti-Ln-332 and anti-BP180 IgG, anti-Ln-332 and anti-BP230 IgG, as well as between anti-Ln-332 and DIF.
Comparison of diagnostic accuracy between anti-Ln-332 IIF and the diagnostic gold standard of DIF was as following: 100% sensitivity, 8% specificity, 21% positive predictive value (PPV), 100% negative predictive value (NPV).
Discussion
The diagnostic procedure of MMP relies on different laboratory investigations, including the histopathological analysis, the detection of tissue-bound immunoreactants by DIF as well as of serum autoantibodies by IIF and molecular immunoassays (ELISA).1 Patients with anti-Ln-332 MMP cannot be phenotypically differentiated from other variants of MMP.12 However, the laboratory diagnosis of anti-Ln-332 is complicated. Specific methods for the detection of anti-Ln-332 autoantibodies, including the immunoprecipitation and immunoblotting, are non-quantitative, laborious, often expensive, tightly regulated and restricted to a few specialized laboratories worldwide. Previously, the immunoprecipitation of radiolabelled cultured keratinocytes has been the gold standard for the detection of anti-Ln-332 autoantibodies in MMP patients.1 However, as was presented by Goletz et al,14,15 the development and introduction of the Ln-332-specific biochip mosaic in 2019 provided available standardized method for the detection of anti-Ln 332 serum circulating autoantibodies. This test showed a satisfactory diagnostic accuracy (84% sensitivity and 99.8% specificity) for anti-Ln-332 MMP patients and controls what was proved in the pioneering study.15 In anti-Ln-332 MMP, antibodies are mainly targeted against α3 or γ2 subunit. Antibodies to β3 subunit are rare; however, they have been revealed in the literature.16 Gasparini et al,17 examining Italian MMP patients with IIF BIOCHIP mosaic, demonstrated that only 6% of patients had anti-Ln-332 antibodies, which is compatible with our results. It was proved that the rather low clinical MMP activity is paralleled by a relatively low autoantibody reactivity in patients peripheral blood. Thus, not unexpectedly, in most of the MMP patients no autoantibodies are detected by IIF microscopy.1 Therefore, various Ln-332 ELISA approaches were postulated to facilitate diagnosing MMP.1,18 Still, Bernard et al18 demonstrated that none of the serum samples from patients whose MMP was graded as low severity (n = 24) showed anti-Ln-332 antibodies with ELISA. For that reason, we speculate that ethnic Poles with MMP examined by us had too low activity of the disease to produce anti-Ln-332 antibodies.
Human IgG antibodies are heterogeneous and consist of 4 subclasses, IgG1-4. Due to the rarity of such disorders, studies regarding the specificity of IgG4 are not available in the literature. In all IgG4-mediated autoimmune disease, some common features are raised: (i) IgG4 subclass autoantibodies block protein–protein interactions instead of causing complement mediated tissue injury, (ii) patients respond favourably to rituximab, (iii) a genetic predisposition – HLA class II genes.19 There are several hypotheses describing the possible cause of the predominance of IgG4 subclass in AIBDs. It is postulated that the response might be as a result of specific genetic factors or due to a continued antigenic stimulation affecting the normal distribution of IgG leading to IgG4 restricted response. Interestingly, van Beek et al20 indicated that IgG4 autoimmune response against α3 chain of Ln-332 may be linked to malignancy. Our observations support the notion that IgG4 autoantibodies may preferentially bind to α3 subunit; however, we did not detect cancer in our patient having such antibodies. Our findings revealed that 20% of MMP patients had associated cancer, which was in line with data of La Placa et al,21 where 18% of MMP cases developed a solid tumour. Importantly, one of our patients developed cancer during the MMP follow-up, which confirms the significance of oncological surveillance.22 Obviously, we present only the experience of a single referral centre, hence the relatively small number of MMP patients. Nonetheless, it should be noted that MMP is a very rare disease with limited numbers of individuals affected, and other works exploring MMP based on a similar population size.21
The modification of classical IIF biochip mosaic may be clinically significant because MMP causes are often misdiagnosed as being false-negative. Previous data14,15 indicated that recombinant Ln-332 is useful in laboratory diagnostics of MMP. Our results confirmed the diagnostic importance of IgG4 detection. This was in line with Goletz et al,15 who showed that the sensitivity of IF for the Ln-332 heterotrimer increased when an anti-IgG4 enriched antitotal IgG conjugate was applied. Therefore, it should be noted that the Euroimmun company has just started to offer the commercial kit for the detection of antibodies to Ln-332, but not containing transfectants of all three chains of Ln-332 as separate substrates, using the fluorescein conjugate to IgG + IgG4. Moreover, our data support previous investigations on other AIBDs – pemphigus and BP.23
It was reported that LAD-1, a shedding extracellular fragment of BP180, which is the autoantigen in lamina lucida type of linear IgA bullous dermatosis (ll LABD)24,25 is the most frequently detected autoantigen in MMP.20 This suggests that there is a molecular relationship between MMP and ll LABD, and that in MMP autoimmunity mediated by Ln-332 has limited pathogenic potential compared to that mediated by LAD-1. As far as diagnostic work-up is concerned, it suggests that performing ELISA and mosaic IIF serological studies on MMP-suspected individuals with just routinely used NC16A epitope of BP180 is not enough to detect autoimmunity, and that traditional unsophisticated ssIIF, somewhat paradoxically, is better suited for this purpose, according to our laboratory experience.
Still, some patients may develop an immune response against various different autoantigens, and it is possible that the unusual autoimmune profile develops as a result of epitope spreading.26 In light of this, additional target antigens, like Ln-332 were recognized in anti-p200 pemphigoid. Interestingly, IgG4 reactivity to Ln-332 by IB was observed in 18% patients with anti-p200 pemphigoid, mainly recognizing the α3 chain.25 Anti-Ln-332 reactivity was confirmed in 70% sera from anti-p200 pemphigoid patients.27 The sera with reactivity to the different antigens are identified in some AIBDs patients with dual autoantibody responses, making the final diagnosis elusive.
IgA-mediated MMP cases with autoantibodies against Ln-332 were reported; however, they are extremely rare.26 Literature data documented that these patients presented conjunctival involvement with multiple bullae at the extremities and were refractory to systemic steroid therapy.28 We did not detect anti-Ln-332 IgA in our MMP patients, which may suggest that they are infrequent and therefore we hypothesize that their evaluation should be performed in only MMP cases showing exclusive IgA deposits in DIF.
Conclusion
Our issue-probing study suggests that the detection of antibodies to Ln-332 chains is occasional in Polish MMP sufferers. Still, it seems that the detecting IgG4 antibodies is important in the diagnosis of MMP. Thus, we agree with suggestions that IgG4 antibodies should be added to the routine panel of antibodies evaluated in studies of cases suspected to have MMP to avoid false-negatives. For cost-effectiveness, we speculate that evaluation of anti-Ln-332 IgA using IIF BIOCHIP Ln-332 mosaic is justified in MMP cases showing exclusive IgA deposits in DIF, which should be experimentally verified in further studies.
Acknowledgments
IIF BIOCHIP Ln-332 mosaic was a gift from Euroimmun, Germany. Parts of this study were presented at: (i) The Online Conference: Controversies in Dermatology 2020 (Dmochowski M. Pemfigoid błon śluzowych: co nowego w patogenezie i terapii?/Mucous membrane pemphigoid: What is new in pathogenesis and therapy?) 17–19 December 2020, Wrocław, Poland; (ii) The Online Educational Course: Mucosal Diseases organized by The Poznań Branch of The Polish Dental Society and Wielkopolska Medical Chamber (Dmochowski M. Pemfigoid błon śluzowych: co skrywa ta nazwa?/Mucous membrane pemphigoid: What is in the name?) 21 May 2021, Poznań, Poland. This study was also undertaken within the framework of activities of the European Reference Network-Skin. We gratefully appreciate Ms. Barbara Jastrzębska, a laboratory diagnostician, for her excellent technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chiorean R, Danescu S, Virtic O, et al. Molecular diagnosis of anti-laminin 332 (epiligrin) mucous membrane pemphigoid. Orphanet J Rare Dis. 2018;13(1):111. PMID: 29980216; PMCID: PMC6035451. doi:10.1186/s13023-018-0855-x
2. Schmidt E, Rashid H, Marzano AV, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - part II. J Eur Acad Dermatol Venereol. 2021;35(10):1926–1948. PMID: 34309078; PMCID: PMC8518905. doi:10.1111/jdv.17395
3. Qian H, Natsuaki Y, Koga H, et al. The second study of clinical and immunological findings in anti-laminin 332-type mucous membrane pemphigoid examined at Kurume University-diagnosis criteria suggested by summary of 133 case. Front Immunol. 2021;26(12):771766. doi:10.3389/fimmu.2021.771766
4. Giurdanella F, Nijenhuis AM, Diercks GFH, Jonkman MF, Pas HH. Keratinocyte footprint assay discriminates antilaminin-332 pemphigoid from all other forms of pemphigoid diseases. Br J Dermatol. 2020;182(2):373–381. PMID: 31090065; PMCID: PMC7027452. doi:10.1111/bjd.18129
5. Sadler E, Lazarova Z, Sarasombath P, Yancey KB. A widening perspective regarding the relationship between anti-epiligrin cicatricial pemphigoid and cancer. J Dermatol Sci. 2007;47(1):1–7. doi:10.1016/j.jdermsci.2007.02.012
6. Rousselle P, Scoazec JY. Laminin 332 in cancer: when the extracellular matrix turns signals from cell Anchorage to cell movement. Semin Cancer Biol. 2020;62:149–165. doi:10.1016/j.semcancer.2019.09.026
7. Amber KT, Bloom R, Hertl M. A systematic review with pooled analysis of clinical presentation and immunodiagnostic testing in mucous membrane pemphigoid: association of anti-laminin 332 IgG with oropharyngeal involvement and the usefulness of ELISA. J Eur Acad Dermatol Venereol. 2016;30(1):72–77. doi:10.1111/jdv.13397
8. Huang C, Chen J. Laminin-332 mediates proliferation, apoptosis, invasion, migration and epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma. Mol Med Rep. 2021;23(1):11. PMID: 33179081; PMCID: PMC7673329. doi:10.3892/mmr.2020.11649
9. Kamaguchi M, Iwata H. The diagnosis and blistering mechanisms of mucous membrane pemphigoid. Front Immunol. 2019;10:34. doi:10.3389/fimmu.2019.00034
10. Ricotti C, Kowalczyk J, Fernandez A, Nousari CH. Mucosal “peeling” biopsy technique for the immunopathologic evaluation of desquamative gingivitis-associated mucous membrane pemphigoid. Arch Dermatol. 2008;144(11):1538. PMID: 19015440. doi:10.1001/archderm.144.11.1538
11. Carey B, Joshi S, Abdelghani A, Mee J, Andiappan M, Setterfield J. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182(3):747–753. PMID: 31021396. doi:10.1111/bjd.18032
12. Suresh L, Kumar V. Significance of IgG4 in the diagnosis of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(3):359–362. PMID: 17344074. doi:10.1016/j.tripleo.2006.12.007
13. Jałowska M, Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, Kaczmarek E, Dmochowski M. Conceptualization and validation of an innovative direct immunofluorescence technique utilizing fluorescein conjugate against IgG + IgG4 for routinely diagnosing autoimmune bullous dermatoses. Cent Eur J Immunol. 2021;46(2):183–190. doi:10.5114/ceji.2021.107028
14. Goletz S, Giurdanella F, Holtsche MM, et al. Comparison of two diagnostic assays for anti-laminin 332 mucous membrane pemphigoid. Front Immunol. 2021;2:773720. PMID: 34899726; PMCID: PMC8657402. doi:10.3389/fimmu.2021.773720
15. Goletz S, Probst C, Komorowski L, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. 2019;180(1):149–156. PMID: 30216412. doi:10.1111/bjd.17202
16. Kanwar AJ, Vinay K, Koga H, Hashimoto T. Mucous membrane pemphigoid with antibodies against β3 subunit of laminin-332: first report from India. Indian J Dermatol Venereol Leprol. 2012;78(4):475–479. doi:10.4103/0378-6323.98079
17. Gasparini G, Cozzani E, Di Zenzo G, et al. Anti-laminin 332 antibody detection using biochip immunofluorescence microscopy in a real-life cohort of Italian patients with mucous membrane pemphigoid. Eur J Dermatol. 2021;26. PMID: 34463277. doi:10.1684/ejd.2021.4104
18. Bernard P, Antonicelli F, Bedane C, et al. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. 2013;149(5):533–540. doi:10.1001/jamadermatol.2013.1434
19. Koneczny I, Yilmaz V, Lazaridis K, et al. Common denominators in the immunobiology of igg4 autoimmune diseases: what do glomerulonephritis, pemphigus vulgaris, myasthenia gravis, thrombotic thrombocytopenic purpura and autoimmune encephalitis have in common? Front Immunol. 2021;11:605214. PMID: 33584677; PMCID: PMC7878376. doi:10.3389/fimmu.2020.605214
20. van Beek N, Kridin K, Bühler E, et al. Evaluation of site- and autoantigen-specific characteristics of mucous membrane pemphigoid. JAMA Dermatol. 2022;158(1):84–89. doi:10.1001/jamadermatol.2021.4773
21. La Placa M, Balestri R, Tartari F, et al. Mucous membrane pemphigoid-associated malignancies: case series and a brief overview of the literature. Dermatol Pract Concept. 2019;9(2):119–125. PMID: 31106014; PMCID: PMC6502303. doi:10.5826/dpc.0902a07
22. Jałowska M, Gornowicz-Porowska J, Dmochowski M. Mucous membrane pemphigoid as a paraneoplastic symptom. Derm Dypl. 2020;11(4):32–35.
23. Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, et al. Accuracy of molecular diagnostics in pemphigus and bullous pemphigoid: comparison of commercial and modified mosaic indirect immunofluorescence tests as well as enzyme-linked immunosorbent assays. Postepy Dermatol Alergol. 2017;34(1):21–27. PMID: 28261028; PMCID: PMC5329104. doi:10.5114/ada.2017.65617
24. Dmochowski M, Hashimoto T, Bhogal BS, Black MM, Zone JJ, Nishikawa T. Immunoblotting studies of linear IgA disease. J Dermatol Sci. 1993;6(3):194–200. PMID: 8136317. doi:10.1016/0923-1811(93)90038-q
25. Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol. 1998;110(3):207–210. PMID: 9506436. doi:10.1046/j.1523-1747.1998.00129.x
26. Hayashi I, Shinkuma S, Shimizu S, et al. Mucous membrane pemphigoid with generalized blisters: igA and IgG autoantibodies target both laminin-332 and type XVII collagen. Br J Dermatol. 2012;166(5):1116–1120. PMID: 22182184. doi:10.1111/j.1365-2133.2011.10776.x
27. Holtsche MM, Goletz S, von Georg A, et al. Serologic characterization of anti-p200 pemphigoid: epitope spreading as a common phenomenon. J Am Acad Dermatol. 2020;84(4):1155–1157. doi:10.1016/j.jaad.2020.07.076
28. Natsuga K, Nishie W, Shinkuma S, et al. Circulating IgA and IgE autoantibodies in antilaminin-332 mucous membrane pemphigoid. Br J Dermatol. 2010;162(3):513–517. PMID: 19751242. doi:10.1111/j.1365-2133.2009.09508.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.