Back to Journals » Cancer Management and Research » Volume 12
A Preliminary Study on the Establishment of the PDTX Model
Authors Liu Y, Zhu YP, Cai MZ, Ke B, Li B, Liu N, Xue Q, Zhan HJ, Deng JY, Zhang L, Hao YP, Wang ZQ, Wang L, Liang H
Received 11 September 2019
Accepted for publication 22 December 2019
Published 17 March 2020 Volume 2020:12 Pages 1969—1979
DOI https://doi.org/10.2147/CMAR.S230668
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Yong Liu,1 Yan-Ping Zhu,2 Ming-Zhi Cai,1 Bin Ke,1 Bin Li,1 Ning Liu,1 Qiang Xue,1 Hong-Jie Zhan,1 Jing-Yu Deng,1 Li Zhang,1 Yan-Peng Hao,2 Zhi-Qiang Wang,2 Li Wang,2 Han Liang1
1Department of Gastroenterology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Centre for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin’s Clinical Research Center for Cancer, Tianjin 300060, People’s Republic of China; 2Nanjing Personal Oncology Biological Technology Co. Ltd., Nanjing 211100, Jiangsu, People’s Republic of China
Correspondence: Han Liang
Department of Gastroenterology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Centre for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin’s Clinical Research Center for Cancer, Huan-Hu-Xi Road, Ti-Yuan-Bei, He Xi District, Tianjin 300060, People’s Republic of China
Tel +86 22 2334 0123-1061
Fax +86 22 2334 0123
Email [email protected]
Objective: The current study aims to explore the establishment of the patient-derived tumor xenograft (PDTX) model.
Materials and Methods: Twenty patients with gastric cancer, 10 males and 10 females, were enrolled in the current study. Firstly, the volume, invasion and metastasis of the xenografts were observed. Subsequently, the correlation between tumor tissues of the PDTX mouse model and the patients’ primary tumor tissues was evaluated by pathological H&E staining and immunohistochemistry.
Results: The results showed that the PDTX models corresponding to 15 of the 20 patients were successfully established, and the success rate of PDTX model establishment was 75%. Furthermore, the PDTX models maintained the differentiation degree, morphological characteristics and structural characteristics of tumor cells.
Conclusion: A PDTX model can be used as a substitute for cancer patients in clinical practice and may be suitable for clinical pharmacodynamic screening and new drug development.
Keywords: gastric tumor, PDTX, xenotransplantation model
Introduction
In recent years, more and more research results have suggested that the patient-derived tumor xenografts (PDTX) model can be successfully established using immunity-deficient mice by inoculating the resected tumor tissue from the patient during an operation. The tumor model established by this method can better maintain the growth environment of tumor cells, the matrix and fibroblasts between tumor cells, and can also maintain the basic morphology of tumors and the structure of their internal vascular network, making the three-dimensional structure of the whole tumor model highly consistent with that of the tumors in clinical patients.1,2 (The application in gastric cancer and the success rate of establishing the model are introduced to explain the significance of this experiment.) In the current study, NCG mice were selected as the host of the xenotransplantation of human primary gastric cancer to establish an experimental animal model that can be passaged and resuscitated and retain the clinical tissue characteristics of primary tumors that is the PDTX model. Details are reported as follows:
Materials and Methods
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the ethics committee of Tianjin Medical University Cancer Institute and Hospital. Written informed consent was obtained from the participants. We confirmed that ethical and legal approval was obtained prior to the commencement of the study, and all experiments were performed following relevant named institutional and national guidelines and regulations.
Experimental Animals
NCG mice were purchased from Nanjing University-Nanjing Institute of Biomedicine, SPF grade, 6–8 weeks old, weighing approximately 20 ± 2 g. All mice were fed in the barrier system, the temperature was 25 ± 2 °C and the humidity was 40–70%. The mice were provided with food and water ad libitum. Before the experiment, all the mice were fed adaptively in the barrier system for 5–7 days.
Reagents and Consumables
The required reagents and consumables included 10% formaldehyde, Leibovitz’s L15 tissue preservation solution, double antibodies, surgical equipment, gauze, etc.
Tumor Specimens
In the current study, all the tumor tissues originated from the resected tumor tissues of patients in Tianjin Cancer Hospital, there were a total of 20 patients (Table 1). Tumor tissue was removed by a clinical surgeon and immediately placed in a tissue preservation solution and sent to the laboratory, then necrosis and residual blood on the surface of the tumor tissue were removed in the clean bench.
 |
Table 1 Basic Data of Patients |
Tumor Transplantation Methods and Passage
Fresh tumor tissue removed through clinical surgery was placed in a medium containing 1% double-antibody, then a tissue scissor was used to cut the tissue into several small pieces, approximately 2 mm × 2 mm × 2 mm in size. After disinfection and the preparation of skin on both of the sides of the mice’s forelimbs, a trocar was used to inject the tumor tissue rapidly into the subcutaneous tissue. The puncture needle was then rotated and removed and a cotton ball was used to clean the oozing of blood on the surface of the mice’s skin to prevent biting because of the residual blood. After tumor formation, the length and width of the tumors were measured regularly and the relative tumor volume (relative tumor volume = 0.5× long diameter × short diameter2) was calculated twice a week on average. In the current study, the criterion for successful transplantation of a tumor in mice is that the volume of the tumors in a mouse should exceed 60 mm3. P2 passage was performed when P1 tumors grew to 500 mm3. There were six mice in each group.
Model Monitoring Indicators
(1) Changes in the mental state, activity, diet and hair color of the mice were observed daily. (2) The growth of the local subcutaneously transplanted tumor in the mice of each group was observed and recorded. (3) The tumorigenic rate and mortality rate of the mice in each group were counted. (4) Mice were euthanized, the transplanted tumor was removed, and the histomorphological characteristics of the tumor specimens from the clinical patients and the transplanted tumors of each generation were observed using H&E staining. (5) The expressions of ck8/18, CK7, ki-67, matrix metalloproteinase 9 (MMP-9), epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor-2 (Her-2) in the tissues of P0 and P1 immune generation mice were detected by immunohistochemistry.
Pharmacodynamic Detection
When the average volume of the tumors in P2 mice reached 100–150 mm3, the mice were randomly grouped: one control group (no treatment), and 10 treatment groups (according to the situation of the subjects, and their recommended chemotherapeutic drugs and/or regimens for testing, synchronized therapy with the corresponding drugs and/or regimens for corresponding subjects was given, all tested drugs were provided by the patients).After administration, the general condition of the mice was observed and recorded daily, tumor volume was measured, and the weight of the tumor-bearing mice was also measured twice a week. After three weeks of continuous observation, a tumor growth curve and an animal weight change curve were drawn, and a PDTX test report was written.
Statistical Analysis
We used the software program SPSS 20.0 (IBM, Chicago, USA) to conduct the statistical analysis. Continuous variables were expressed as mean± SD. Discontinuous variables were expressed as percentage (%). A value of P<0.05 was considered statistically significant.
Results
Tumor Formation and Growth of Mice
The primary transplantation of tumor specimens to NCG mice was performed in 20 patients. At 4–6 weeks after inoculation, a slightly rigid solid mass protruding from the skin could be seen at the site of the inoculation. As time progressed, the tumor nodules gradually enlarged. When the tumor volume of the mice exceeded 60 mm3, it indicated that the model was successfully established. The results of the current study revealed that a total of 15 mice were successfully modeled. The success rate of the primary transplantation was 75%. The tumor formation time was 40–115 days. The growth of P1 generation tumors is shown in Figure 1.
Histopathological Examination of Specimens of Patients and Transplanted Tumors
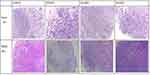
When the tumor volume in mice increased to 800 mm3, the mice were euthanized and the transplanted tumors removed. The results of the current study reveal that the transplanted tumors in the mice were smooth on the surface, round or quasi-round in shape, solid, and slightly hard in texture. The tumors were cut according to the P1 generation inoculation method and re-injected into P2 generation mice. No obvious infiltrations were found in the other organs of the mice. The results of the H&E staining of the specimens of clinical patients and the expressions of ck8/18, CK7, Ki-67 and MMP-9 in tumor tissues are shown in Figures 2 and 3. Moreover, the histopathological structure of PDTX mice tumors was basically consistent with that of the corresponding patient.
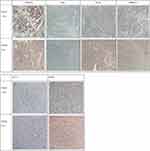 |
Figure 3 The expressions of ck8/18, CK7, ki-67 and MMP-9 in P0 and P1 tissues. The expressions of ck8/18, CK7, Ki-67 and MMP-9 are significant in tumor tissues in clinical patients. |
Pharmacodynamic Test Results
The pharmacodynamics test was carried out in 0501011 patients after successful modeling. As shown in Figure 4, the results revealed that Scheme 6 (gemcitabine) > Scheme 11 (cisplatin + tegafur) > Scheme 8 (cisplatin) > Scheme 3 (irinotecan) > Scheme 2 (oxaliplatin + tegafur) > Scheme 9 (albumin paclitaxel + capecitabine) > Scheme 7 (albumin paclitaxel). The tumor growth inhibition value (TGI) and relative tumor volume (RTV) of these drugs were listed in the Table 2. When the PDTX model was established, the patient began to receive clinical concurrent adjuvant chemotherapy after the operation, and the treatment regimen was oxaliplatin + tegafur. Because the side effects of tegafur are substantial in clinical treatment, the drugs were adjusted to oxaliplatin + retitroxel (currently, a clinical follow-up is underway).
 |
Table 2 Anti-Tumor Rate of Different Chemotherapy Regimens |
Clinical Follow-Up of Prognosis
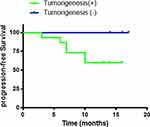
The duration of the clinical follow-up was 17 months. Follow-up results revealed that, of the 15 patients whose models were successfully established, disease progression during the follow-up period occurred in six patients. Disease in the five patients whose models were unsuccessfully establishedisstable at present. The success rate of the PDTX model correlates significantly with the prognosis of the patient, which is worth further study (p=0.0086, <0.05). The prognoses of clinical patients during follow-up are shown in Figure 5.
Discussion
At present, epidemiological data reveals that the morbidity and mortality of gastric cancer ranks first among malignant digestive system tumors in China. Although at present surgery, radiotherapy and chemotherapy are still the first choices for the treatment of gastric cancer, the results of a large number of studies have revealed that there were significant individual differences in biological characteristics and drug sensitivity of tumor tissues in clinical patients with tumors, even if the same type of cancer has huge individual differences among different patients. At present, the development of new anti-cancer drugs and the selection of clinical chemotherapy are often based on the drug sensitivity test of tumor cells cultured in vitro, genetic testing and in vivo models of the transplantation of passaged tumor cells, but these do not really reflect the individual’s sensitivity to chemotherapeutic drugs or targeted drugs, therefore, the predictability was poor.1,2 In recent years, some foreign scholars used a PDTX model for pharmacodynamics tests, which provided a new idea for individualized treatment of clinical cancer patients.
The results of previous studies have revealed that the success rate of PDTX modeling for surgical specimens was 25–40%.3–5 It was also reported that the success rate of needle aspiration puncture for small cell lung cancer was 75%.6 In the current study, among these 20 patients, a PDTX model was successfully established for 15 patients, making the success rate of modeling 75%, with the PDTX model maintaining the biological characteristics of the primary tumors. The result is consistent with that of previous studies on, gastric cancer, liver cancer, lung cancer, colorectal cancer, melanoma, and esophageal cancer etc. Therefore, a PDTX model is a stable and reliable cancer research model andcan provide a good animal model for cancer studies.7–9
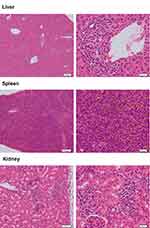
In the current study, a mice model was unsuccessfully established for five patients. The main reasons are as follows: 1. accidental death, 2. tumors did not grow, or they grew slowly and then did not meet the criteria, 3. infection. During the process of this experiment, one month after inoculation, for specimens A19078, A19080, 0501006 and 0501007, some mice started to successively develop hair loss and became weak. After examination, no microbial infection was found, inflammatory cell infiltration was found in the portal area of the liver, perivascular area and renal interstitium, vigorous karyokinesis were found in some cells in the spleen, and no obvious pathological changes were found in the ovaries and uterus (Figure 6). Because the team of this study has carried out immune reconstitution experiments in NCG mice, similar phenomena occurred one month after tail vein injection of peripheral blood mononuclear cell (PBMC), therefore, it was considered that the reason may be related to immune rejection. It is suspected that it is caused by further inflammatory cell infiltration in the inoculated tumor tissue. Its mechanism needs further study.
The results of this study revealed that the characteristics of blood supply, matrix, growth and necrosis of the transplanted tumors in the mice were consistent with those of human tumors. Therefore, PDTX modeling is likely to be an important and highly homogenous in vivo model. At present, the tissue source for the PDTX model was mainly resected tumor tissue during enlarged surgery. However, many patients with advanced tumors are not suitable for surgical treatment and tumor tissues cannot be obtained from these patients, therefore, the clinical application of PDTX is limited.
In order to confirm that P1 generation tumors have the same proliferation activity and malignant behavior as primary tumors, its proliferation potential and transfer risk are consistent, and mutation sites and gene expressions can be an effective alternative, the expressions of ck8/18, CK7, ki-67, MMP-9, EGFR and Her-2 in P0 and P1 tissues were selected and analyzed. The results revealed that the two types of tissues were highly homogenous. Cytokeratin (CK) is an intermediate fiber; its family consists of highly complex polygenic proteins, and the molecular weight ranges between 40–68 kDa.Up until now, 20 CK proteins (CK1-CK20) have been discovered. An in vitro experiment revealed that, compared with the control cells, various cell lines overexpressing CK8/18, including lung adenocarcinoma, melanocytes and mouse L cell lines, have a higher migration and stronger invasiveness. ki-67 is a relatively positive marker for nuclear proliferation in relation to cell cycle, which only exists in the nucleus of proliferating cells.10 The coding gene is located on chromosome 10 and its expression increases with the progress of cell cycle. In the cell division cycle, it is not expressed in G0 phase, begins to express in G1 phase, increases gradually in S and G2, reaches its peak in M phase, and degrades and disappears rapidly after the end of cell division, therefore it is an important indicator of cell proliferative activity, and widely used to evaluate the proliferation of cancer cells. There have been many studies on the relationship between gastric cancer and Ki-67, but there is still controversy at present. Studies have revealed that the expression of Ki-67 in gastric cancer tissues was closely correlated to the clinicopathology and prognosis of the patients. Tsamandas et al revealed in their studies that ki-67 was correlated to the prognosis of patients with gastric cancer. Kikuyama et al considered that Ki-67 was an independent prognostic factor of patients with gastric cancer. Matrix metalloproteinases (MMPs) are proteolytic enzymes very closely related to invasion and metastasis of tumors. As a matrix, metalloproteinase can degrade basement membrane and extracellular matrix components, and accordingly, promote the infiltration and metastasis of tumors and interstitial angiogenesis. The detection of its expression level plays an important role in the prospective determination of the biological characteristics of tumors and it acts as a prognostic indicator. In the current study,CK8/18 and Ki-67 were highly expressed in all three patients whose models were successfully established, whether in the original tumors or in the PDTX model. The results suggest that tumor tissue has strong invasiveness and metastasis ability. MMP-9 was weakly positive in the model of one patient. Its significance in predicting the prognosis of gastric cancer needs further verification.
In the PDTX study on colorectal cancer published by Samsung Medical Center in South Korea,11 241 patients with colorectal cancer were analyzed retrospectively. The model of nude mice was established by transplanting surgical samples to investigate the relationship between the success rate of PDTX and clinical prognosis in patients with colorectal cancer. The results revealed that the prognosis of patients whose model was successfully established poorer than that of patients whose model was not successfully established, especially obvious in patients at stage 3, long-term differences could also be seen in patients at stage 4 with metastasis. The prognosis of patients whose model was successfully established was poorer than that of patients whose model was not successfully established. This suggests that PDTX can reflect the invasiveness of tumors and can be used as one of the indicators to predict recurrence and metastasis in the future. In the current study, 1-year follow up results revealed that of the 15 patients whose models were successfully established, disease progression occurred in six patients, while disease in patients with a failed model establishment is currently stable. The success rate of PDTX models was significantly correlated with the prognosis of patients, which is worth further study.
In our study, the pharmacodynamics test revealed that Scheme 6 (gemcitabine) > Scheme 11 (cisplatin + tegafur) > Scheme 8 (cisplatin) > Scheme 3 (irinotecan) > Scheme 2 (oxaliplatin + tegafur) > Scheme 9 (albumin paclitaxel + capecitabine) > Scheme 7 (albumin paclitaxel). However, the specific differences among these kinds of chemotherapy regimens should be further research in the future.
PDTX models can better preserve the biological characteristics of primary tumor tissues and their response to drugs.12–16 PDTX models can be used as a substitute for cancer patients in clinical practice and may be suitable for clinical pharmacodynamic screening and new drug development. It has a wide application prospect.17
Funding
The study was funded by the National Key Research and Development program “precision medicine research” program (2017YFC0908300).
Disclosure
Dr Zhu and Dr Hao are the employees of the company Nanjing Personal Oncology Biological Technology Co. Ltd. The authors report no other conflicts of interest in this work.
References
1. Sjöblom T, Jones S, Wood LD, et al. The Consensus Coding Sequences of Human Breast and Colorectal Cancers. Science. 2006;314:268–274. doi:10.1126/science.1133427
2. Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18:5160–5162. doi:10.1158/1078-0432.CCR-12-2408
3. Zhang XC, Zhang J, Li M, et al. Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies. J Transl Med. 2013;11:168. doi:10.1186/1479-5876-11-168
4. Fichtner I, Rolff J, Soong R, et al. Establishment of patient-derived Non-small cell lung cancer xenografts as modeles for the identification of predictive biomarkers. Clin Cancer Res. 2008;14:6456–6468. doi:10.1158/1078-0432.CCR-08-0138
5. John T, Kohler D, Pintilie M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage Non-small cell lung cancer. Clin Cancer Res. 2011;17:134–141. doi:10.1158/1078-0432.CCR-10-2224
6. Anderson WC, Boyd MB, Aguilar J, et al. Initiation and Characterization of Small Cell Lung Cancer Patient-Derived Xenografts from Ultrasound-Guided Transbronchial Needle Aspirates. PLoS One. 2015;10:e0125255. doi:10.1371/journal.pone.0125255
7. Hao C, Wang L, Peng S, et al. Gene mutations in primary tumors and corresponding patient-derived xenografts derived from non-small cell lung cancer. Cancer Lett. 2015;357(1):179–185. doi:10.1016/j.canlet.2014.11.024
8. Zhu Y, Tian T, Li Z, et al. Establishment and characterization of patient-derived tumor xenograft using gastroscopic biopsies in gastric cancer. Sci Rep. 2015;5:8542. doi:10.1038/srep08542
9. Gu Q, Zhang B, Sun H, et al. Genomic characterization of a large panel of patient-derived hepatocellular carcinoma xenograft tumor models for preclinical development. Oncotarget. 2015;6:20160–20176. doi:10.18632/oncotarget.3969
10. Nowicki M, Ostalska-Nowicka D, Miśkowiak B. Prognostic significance of Ki-67 negative blaste cell clone in the high risk group of children treated for acute myeloid leukaemi. Folia Histochem Cytobiol. 2006;44:49–52.
11. Malaney P, Nicosia SV, Davé V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 2014;344:1–12. doi:10.1016/j.canlet.2013.10.010
12. Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi:10.1038/nrclinonc.2012.61
13. Julien S, Merino-Trigo A, Lacroix L, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18:5314–5328. doi:10.1158/1078-0432.CCR-12-0372
14. Scott CL, Becker MA, Haluska P, Samimi G. Patient-derived xenograft models to improve targeted therapy in epithelial ovarian cancer treatment. Front Oncol. 2013;3:295. doi:10.3389/fonc.2013.00295
15. Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73:5315–5319. doi:10.1158/0008-5472.CAN-13-1069
16. Liu Q, Zheng L, Zhang H. The research progress of patient-derived tumor xenografts. Transl Med J. 2014;3(02):65–68.
17. Rosfjord E, Lucas J, Li G, Gerber HP. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91:135–143. doi:10.1016/j.bcp.2014.06.008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.