Back to Journals » Infection and Drug Resistance » Volume 14
A Preliminary Exploration of the Efficacy of Gentamicin Sponges in the Prevention and Treatment of Wound Infections
Received 9 April 2021
Accepted for publication 3 June 2021
Published 8 July 2021 Volume 2021:14 Pages 2633—2644
DOI https://doi.org/10.2147/IDR.S315105
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yongduo Li, Junlin Zhou
Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China
Correspondence: Junlin Zhou
Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, No. 8 of Gongren Tiyuchang Nanlu, Chaoyang District, Beijing, 100020, People’s Republic of China
Tel/Fax +86-13240718193
Email [email protected]
Purpose: This study aimed to construct drug-loading and drug-releasing quantitative equations for gentamicin sponges in addition to realizing a gentamicin sponge for wound infection prevention and treatment.
Methods: Sterile sponges were cut into pieces of 1× 1 × 0.5 cm and immersed in 40, 16, 8, 4, 1.6, 0.8, or 0 mg/mL of gentamicin solution for 12, 24, 48, 96, or 120 h to evaluate their gentamicin loading. The sponges were subsequently immersed in the gentamicin solution of different concentrations for 48 h, air-dried, and then immersed in 10 mL of 0.9% physiological saline to evaluate the gentamicin release. Methicillin-sensitive Staphylococcus aureus (MSSA) and Pseudomonas aeruginosa were used to explore the sponges’ infection prevention scheme. In addition, a rat femur fracture with wound infection model was used to assess the infection treatment scheme.
Results: The antibacterial zone sizes of the sponges immersed in 40, 16, 8, 4, 1.6, and 0.8 mg/mL of the gentamicin solution were larger than those of the 0 mg/mL air-dried sponge, and the difference was statistically significant (p < 0.01, p < 0.01, p < 0.01, p < 0.01, p < 0.01, and p < 0.01, respectively). The rats in the 40, 16, and 8 mg/mL air-dried sponge groups had no wound suppuration in either the MSSA or P. aeruginosa rat infection models.
Conclusion: A quantified equation for the sponges’ gentamicin loading and release was achieved with high accuracy. Furthermore, we recommend the 40, 16, or 8 mg/mL air-dried sponge for the treatment of wounds with antibiotic-sensitive bacterial infections.
Keywords: gentamicin sponge, quantitative equation, prevention concentration, treatment scheme, in vitro and in vivo experiments
Introduction
Gentamicin sponges, implantable topical antibiotic agents, are approved for surgical implantation in 54 countries. Since 1985, more than one million patients have been treated with these sponges.1–3 However, despite having been studied for over 30 years, their effectiveness is still disputed.1,4–8
Han et al have found that applying gentamicin-impregnated sponges during spinal operations significantly decreases surgical-site infection (SSI).4 Chang et al have conducted a meta-analysis encompassing 15 randomized control trials and also concluded that gentamicin sponges decrease the rate of SSI.5 Schimmer et al have used a controlled, prospective, randomized double-blind study to investigate the efficacy of gentamicin sponges in sternal wound complications after heart surgery. They enrolled 720 patients and found that gentamicin sponges effectively reduce infection complications.6
However, several other studies have demonstrated that gentamicin-impregnated sponges cannot reduce SSI, and some researchers have even proposed that the sponges increase the risk of infection. Wouthuyzen-Bakker et al have discussed the efficacy of applying gentamicin-impregnated sponges locally during debridement in early acute periprosthetic joint infections. They found that their application does not reduce the incidence of infection and, thus, discourage their use.7 Uçkay et al have evaluated the benefits of using gentamicin sponges to treat mild diabetic foot ulcer infection. Regrettably, although the sponges achieved very good tissue tolerance, they did not improve the infection outcome.8 Bennett-Guerrero et al have found that gentamicin sponges increase the rate of SSI among patients undergoing open or laparoscopically assisted colorectal surgery;1 many other articles have noted similar results.
Overall, there are still debates about the anti-infection effectiveness of gentamicin sponges as well as several discrepancies in the previous studies. First, the amount of gentamicin contained in the sponges was inconsistent in each study and each surgical implantation approved by the various different countries, which may have resulted in conflicting conclusions. Second, the sizes of the sponges used in the different studies were also different, and there are no uniform standards or use guidelines for gentamicin sponges. Third, the existence of a local infection before using the gentamicin sponges was inconsistent. In some studies, the sponges were used in aseptic surgery to explore their preventive effect in relation to infection, but, in others, they were used in infected patients or animals to analyze their therapeutic effect. Fourth, the observation time points were not consistent.
The purpose of this study is to investigate the effect of gentamicin sponges in vitro and in vivo. First, the gentamicin loading of the sponges is analyzed to construct a loading equation. Second, the release of gentamicin from air-dried gentamicin-saturated sponges is evaluated to obtain the release equation. Third, the antibacterial effect of the sponges is observed in vitro. Fourth, the anti-infection and mortality-reduction effects of the sponges are explored in a rat femur fracture with wound infection model. Finally, recommendations are outlined for the clinical application of gentamicin sponges, especially for patients with open fracture infections.
Methods
Reagents
Luria broth (LB) powder (containing 10 g of peptone, 5 g of yeast powder, and 10 g of NaCl) was purchased from Beijing Solarbio Science & Technology Co., Ltd., and phosphate-buffered saline (PBS) was purchased from Merck KGaA. The LB medium was made by dissolving the powder in 1 L of sterile PBS, filtering with 0.22 μm, and then freezing it at 4°C. Sterile physiological saline (0.9%) was purchased from Baxter Medical Products Co., Ltd., and sodium pentobarbital was provided by the Medical Research Center of Beijing Chaoyang Hospital, affiliated with Capital Medical University. A gentamicin enzyme-linked immunosorbent assay (ELISA) kit was purchased from CUSABIO Bioengineering Co., Ltd.
Bacterial Strain
We used two kinds of bacteria: methicillin-sensitive Staphylococcus aureus (MSSA) and Pseudomonas aeruginosa. In addition, we analyzed the effects of gelatin gentamicin on MSSA and P. aeruginosa in in vitro and animal experiments and did not involve clinical research. The next step is to carry out clinical studies on MSSA biofilms.
The MSSA (ATCC 25923) standard strain and P. aeruginosa (ATCC 27853) were provided by the Guangdong Microbial Species Preservation Center.
Animals
Female adult Sprague Dawley rats (Charles River Laboratories, 200 ± 20 g) were kept in separate cages under a 12-h light/dark cycle at 23.6°C and 35% humidity. The animals were fed a sterilized chow diet and water. All procedures complied with the Animal Research: Reporting of In Vivo Experiments guidelines and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The present study was approved by the Capital Medical University ethics committee on the use of animals in research and education. At the end of the experiments, the rats were euthanized via an excessive dose of sodium pentobarbital (100 mg/kg, intraperitoneal injection). Our ethical code for animal experiments is AEEI-2020-192.
Rat Femur Fracture with Wound Infection Model
The detailed process was described as follows.. In brief, we built a MSSA and P. aeruginosa rat wound infection model with a femur fracture. The LB medium was used to dissolve bacterial strain powders, and the bacteria suspension was cultured at 37°C and 200 rpm/min overnight. The precipitate was acquired at 10,000 rpm/min for three minutes and then resuspended in LB medium. After the bacterial passage was performed three times, the bacterial suspension was diluted 10-fold by the sequential transfer of 100 μL into 900μL of PBS. Then, 100 μL of the different diluted bacterial suspensions was inoculated into different LB agar media at 37°C overnight. The number of colonies yielded was between 20 and 200. The colonies were counted and multiplied by the corresponding dilution times to obtain the suspensions’ bacterial quantities, which were adjusted to 106, 107, 108, and 109 CFU/mL using the LB medium.
The rat femur fracture model was established on a small-animal operating table on a clean bench. We referred to Husodo et al9 for this model. Briefly, the rats were fasted for 12 h and not permitted to drink water for 3 h before anesthesia. Isoflurane (concentration of 3%, oxygen flow at 3 L/min, small-animal anesthesia machine) was used to induce the anesthesia, which was maintained by sodium pentobarbital (0.4 mL/100 g, 40 mg/kg, intraperitoneal injection). The right hind limb of the rats was shaved and disinfected three times with 75% alcohol. The right lateral position was adopted, and the right hind limb was draped with a sterile surgical towel. The femoral lateral approach was used. The incision was 1–1.5 cm long. The skin, subcutaneous tissue, and superficial and deep fascia were opened, and the middle part of the femur was exposed through the tensor fascia lata and femoral lateral muscle gap. A bone saw was used to create a femur shaft transverse fracture. Kirschner wire (1.0 mm) was used to fix the fracture as an intramedullary nail. The movement of the hip and knee and contraposition, alignment, and fixation of the fracture were examined. The method used to cause the bacterial infection was as described in a previous study.10 In brief, the bacterial suspension was administered to the rats by gradually dripping it onto the surface of the muscle and embrocating with a sterile bacterial inoculation needle. The bacterial suspension volume was 0.5 mL at concentrations of 106, 107, 108, and 109 CFU/mL. The muscle and skin incision was sutured using a 3–0 silk suture. The rats were kept in separate cages with a sterilized chow diet and water.
The mortality rate and wound tissue sections of the rats were obtained following the model’s establishment to evaluate whether the model had succeeded and was stable Finally, we chose 108 CFU/mL of MSSA and 106 CFU/mL of P. aeruginosa to establish the rat model (related results are in the supplementary material: DOI:10.17632/vpj7vjkz86.1).
Evaluation of the Gentamicin Loading of the Sponge
Gentamicin powder was dissolved in 0.9% physiological saline, and we adjusted the concentration to 40, 16, 8, 4, 1.6, 0.8, or 0 mg/mL. Gentamicin sponges were cut into pieces of 1×1 × 0.5 cm and sterilized with ethylene oxide (Figure 1). These sponges were then divided into seven groups according to the concentration of gentamicin solution and immersed in solutions of different concentrations for 12, 24, 48, 96, or 120 h. The sponges were then air-dried until there were no further changes (about 48 h). The sponges’ weight was measured by an electronic balance to evaluate the gentamicin loading.
 |
Figure 1 Gentamicin sponge cut into pieces of 1×1 × 0.5 cm. |
Preparation of the Air-Dried Sponge
We used similar methods to the above to prepare the air-dried sponges. In brief, gentamicin powder was dissolved in 0.9% physiological saline, and the concentration was adjusted to 40, 16, 8, 4, 1.6, 0.8, or 0 mg/mL. The sponges were cut into pieces of 1×1 × 0.5 cm and sterilized with ethylene oxide. They were then divided into seven groups according to the concentration of the gentamicin solution, immersed in solutions of different concentrations for 48 h, and air-dried until there were no further changes (about 48 h). Finally, they were sterilized with ethylene oxide and kept at –20°C until use.
Evaluation of the Drug Release of the Air-Dried Gentamicin-Saturated Sponges
The air-dried sponges were immersed in 10 mL of 0.9% physiological saline for 0.5, 2, 6, 12, 24, 48, and 96 h. The gentamicin ELISA kit was used to test the concentration in 10 mL of 0.9% physiological saline. The specific operation steps were in accordance with the manual.
Evaluation of the Antibacterial Effect of a Gentamicin Sponge in vitro
After bacterial passage was performed three times, as described previously, 100 µL of bacterial suspension was inoculated into LB agar media at 37°C and left overnight so that the surface of the medium was fully covered in colonies. An air-dried sponge (1×2×0.5 cm size) was placed in the center of the LB agar media surface at 37°C for 48 h. The sponge was then removed, and the antibacterial area (where bacteria did not grow) was measured to evaluate the sponge’s antibacterial effect.
Evaluation of the Effects of Gentamicin Sponges on the Rat Femur Fracture with Wound Infection Model
Here, 70 rats were divided into two groups to form the MSSA infection group (35 rats) and P. aeruginosa infection group (35 rats). Each group was then divided into seven subgroups: 40 mg/mL air-dried sponge (five rats), 16 mg/mL air-dried sponge (five rats), 8 mg/mL air-dried sponge (five rats), 4 mg/mL air-dried sponge (five rats), 1.6 mg/mL air-dried sponge (five rats), 0.8 mg/mL air-dried sponge (five rats), and 0 mg/mL air-dried sponge (five rats). As described previously, we chose 108 CFU/mL of MSSA and 106 CFU/mL of P. aeruginosa to establish the rat femur fracture with wound infection model. After the bacterial suspension was administered to the rats, an air-dried sponge was placed in the bacteria-inoculated muscle gap, and the muscle and skin incision was sutured using a 3–0 silk suture. The rats were kept in separate cages with a sterilized chow diet and water. The rats’ mortality and wound suppuration rate were recorded at day seven.
Statistical Analysis
The SPSS 23.0 software was used to analyze the data. The measurement data were reported as the median (interquartile range); the Kruskal–Wallis H-test was used to compare multiple groups, and the Mann–Whitney U-test was used to compare two groups. The enumeration data were given as percentages; an χ2 test and Fisher’s definite probability methods were used to compare groups. A P-value <0.05 was considered statistically significant.
The equations for the gentamicin loading of the sponge and drug release of the air-dried gentamicin-saturated sponge were constructed by curve estimation using the SPSS software. The R2 coefficient of determination was used to evaluate the fit of the constructed equation. We used the median of each group at each time point to construct the surface estimation in the Origin 2019b software. The maximum number of iterations was set at 400, the tolerance was 1e-9, and the iterative algorithm was the Levenberg–Marquardt algorithm. The surface function was set to Poly2D, and the coefficients of the surface equation were expressed by the mean ± standard deviation. Additionally, R2 was used to evaluate the fit of the constructed equation, and Microsoft Excel 2016 was used for the data visualization of the wound suppuration rate.
Results
Evaluation of the Gentamicin Loading of the Sponges
We compared the original weight of the air-dried sponges and, then, the difference in weight between the 1.6 and 0.8 mg/mL air-dried sponges. As the sponge began to decompose when it was immersed in the solution for 96 h, the weight of the 0 mg/mL air-dried sponge at 96 and 120 h was lower than the original weight (p = 0.01 and p = 0.01, respectively).
Next, we compared the amount of gentamicin released from the sponges, which increased gradually over time; the difference was statistically significant (p < 0.01). Moreover, the amount of gentamicin released from the sponges immersed in the gentamicin solution of different concentrations also differed (p < 0.01). The amount of gentamicin released from the sponge immersed in the 40 mg/mL gentamicin solution was the largest at each time point, and the difference was statistically significant (p < 0.01).
Then, we compared the antibacterial zone sizes. The 1.6 and 0.8 mg/mL air-dried sponges demonstrated no statistical differences for either P. aeruginosa or MSSA (p = 0.344, p = 0.917, respectively). Furthermore, the antibacterial zone sizes of the sponges previously immersed in 40, 16, 8, 4, 1.6, and 0.8 mg/mL of gentamicin solution were larger than those of the 0 mg/mL air-dried sponge, and the difference was statistically significant (p < 0.01).
Finally, we evaluated the effects of the gentamicin sponges on the rats with femur fractures and wound infections.
There were no deaths by day seven of any rats with femur fractures and wound infections. The wound suppuration results of the rats with the P. aeruginosa infection are shown in Figure 2. The animals in the 40 (five rats), 16 (five rats), and 8 mg/mL (five rats) air-dried sponge groups had no wound suppuration; thus, their wound-suppuration rates were 0. Although the 4 (five rats, one with wound suppuration), 1.6 (five rats, one with wound suppuration), and 0.8 mg/mL (five rats, one with wound suppuration) air-dried sponge groups contained rats with wound suppuration, the differences between these groups and the 40 mg/mL air-dried sponge group were not statistically significant (p > 0.99, p > 0.99, and p > 0.99, respectively). While the wound suppuration rate for the 0 mg/mL air-dried sponge group (five rats) was significantly higher than that of the 4 (p = 0.048), 1.6 (p = 0.048), and 0.8 mg/mL (p = 0.048) groups, it was not significantly different from the 40 (p > 0.99), 16 (p > 0.99), or 8 mg/mL (p > 0.99) groups.
 |
Figure 2 Wound suppuration rates of the rats with P. aeruginosa wound infection. *p < 0.05 compared with the 40 mg/mL air-dried sponge group. |
The gentamicin loading of the sponges is shown in Table 1. There was no statistical difference in the original weight of the air-dried sponges (p = 0.92). However, when the immersion time in the gentamicin solution was prolonged, the difference became statistically significant in multiple groups. The sponge immersed in 40 mg/mL of gentamicin solution had the highest weight. The difference in weight between the 1.6 and 0.8 mg/mL air-dried sponges was not statistically significant at any of the observation time points (p > 0.05). As the sponges began to decompose when immersed in the solution for 96 h, the weight of the 0 mg/mL air-dried sponge at 96 and 120 h was lower than the original weight (p = 0.009 and p = 0.009, respectively).
 |
Table 1 The Gentamicin-Loading for Each Sponge Type |
The equation for the gentamicin loading of the sponges is shown in Table 2. Here, R2 was used to evaluate the fit and, for all constructed equations, was above 0.9. The Origin 2019b software was used to construct the surface equation of the sponge gentamicin loading:
 |
Table 2 The Equation of Gentamicin-Loading by Sponge Type |
z = (0.03718 ± 0.01672)x + (–4.578e-4 ± 0.06253)y + (–2.50935e-4 ± 1.47521e-4)x2 + (0.00303 ± 0.00149)y2 + (0.00408 ± 3.52827e-4)xy,
where z is the gentamicin loading of the sponge, x is the duration of the sponge’s immersion in the gentamicin solution, and y is the concentration of the gentamicin solution the sponge was immersed in. The R2 of the surface equation was 0.97 (Figure 3).
Evaluation of Drug Release from the Air-Dried Gentamicin-Saturated Sponges
The results of the drug release from the air-dried gentamicin-saturated sponges are shown in Table 3. Since the sponges began to decompose after being immersed in water for more than 96 h, the longest observation time was set as 96 h. The results showed that the amount of gentamicin released from the sponges increased gradually over time and that the difference was statistically significant (p < 0.01). Moreover, the amount of gentamicin released from the sponges immersed in the gentamicin solution of different concentrations also differed (p < 0.01). The amount of gentamicin released from the sponge immersed in the 40 mg/mL gentamicin solution was the largest at each time point, and the difference was statistically significant (p < 0.01).
 |
Table 3 The Drug-Release of Different Types of Air-Dried Gentamicin-Saturated Sponges |
The equation for the drug release from the air-dried gentamicin-saturated sponge is shown in Table 4. The R2 for all constructed equations was above 0.9 (0.9 indicates that 90% of the variance in the dependent variable is explained by the variance in the independent variable). The surface equation for the gentamicin release from an air-dried sponge was
 |
Table 4 The Equation of Gentamicin-Release by Sponge Type |
z = (4.37205 ± 1.18048)x + (–7.05921±3.09628)y + (–0.04596 ± 0.01287)x2 + (0.3309 ± 0.07912)y2 + (0.31559 ± 0.02754)xy,
where z is the gentamicin release from the air-dried sponge, x is the duration of the sponge’s immersion in the gentamicin solution, and y is the concentration of the gentamicin solution the sponge was immersed in. The R2 of the surface equation was 0.95 (Figure 4).
Evaluation of the Antibacterial Effect of the Gentamicin Sponge in vitro
The antibacterial zone size of the gentamicin sponges showed that the air-dried sponges previously immersed in 40 mg/mL of gentamicin solution had a larger antibacterialzone size than the others for P. aeruginosa (p < 0.01). The antibacterial zone sizes of the 1.6 and 0.8 mg/mL air-dried sponges demonstrated no statistical differences for either P. aeruginosa or MSSA (p = 0.344, p = 0.917, respectively). Furthermore, the antibacterial zone sizes of the sponges previously immersed in 40, 16, 8, 4, 1.6, and 0.8 mg/mL of gentamicin solution were larger than those of the 0 mg/mL air-dried sponge, and the difference was statistically significant (p < 0.01).
Evaluation of the Effects of the Gentamicin Sponges on Rats with Femur Fractures and Wound Infections
There were no deaths by day seven of any of the rats with femur fractures and wound infections. The wound suppuration results of the rats with the P. aeruginosa infection are shown in Figure 2. The rats in the 40 (five rats), 16 (five rats), and 8 mg/mL (five rats) air-dried sponge groups had no wound suppuration; thus, their wound suppuration rates were 0. Although the 4 (five rats, one with wound suppuration), 1.6 (five rats, one with wound suppuration), and 0.8 mg/mL (five rats, one with wound suppuration) air-dried sponge groups contained rats with wound suppuration, the differences between these groups and the 40 mg/mL air-dried sponge group were not statistically significant (p > 0.99, p > 0.99, and p > 0.99, respectively). While the rate of wound suppuration for the 0 mg/mL air-dried sponge group (five rats) was significantly higher than that of the 4 (p = 0.048), 1.6 (p = 0.048), and 0.8 mg/mL (p = 0.048) groups, it was not significantly different from the 40 ((p = 0.008), 16 (p = 0.008), or 8 mg/mL (p = 0.008) groups.
The wound suppuration rates of the rats with the MSSA infection are shown in Figure 5. The animals in the 40 (five rats), 16 (five rats), 8 (five rats), and 4 mg/mL (five rats) air-dried sponge groups had no wound suppuration; thus, their wound suppuration rates were 0. However, the 1.6 (five rats, one with wound suppuration) and 0.8 mg/mL (five rats, one with wound suppuration) air-dried sponge groups contained rats with wound suppuration. The differences between the 1.6 and 40 mg/mL air-dried sponge groups were not statistically significant (p > 0.99). The wound suppuration rate in the 0 mg/mL air-dried sponge group (five rats) was 100%, much higher than that in the other groups (all comparisons between these groups were p > 0.99).
 |
Figure 5 Wound suppuration rates of the rats with MSSA wound infection. *p < 0.05 compared with the 40 mg/mL air-dried sponge group. |
Discussion
Gentamicin sponges have been studied for over 30 years, but their efficacy remains controversial.1,11–15 Many researchers have demonstrated that the application of gentamicin sponges can significantly reduce the incidence of wound infection and kill pathogenic bacteria in both animal experiments and clinical studies.5,16,17 However, there are also many scholars who believe that gentamicin sponges have no effect in the prevention or treatment of wound infections and that these sponges may in fact have the opposite effect and increase the incidence of infection.1,7,8,18,19 In particular, a study with a large sample size was previously conducted to confirm these opinions.1 Thus, the effectiveness of the various sizes or drug loading of gentamicin sponges remains unclear.
Gentamicin is an aminoglycoside antibiotic with a clear spectrum of sensitive or resistant bacteria.20,21 If a wound is infected with bacteria, bacterial resistance spectrum screening should be carried out first. Gentamicin sponges are considered to be used for only bacteria that are sensitive to gentamicin; therefore, in this study, we chose two kinds of bacteria that are sensitive to gentamicin. At present, the controversy over the efficacy of gentamicin sponges is related to their drug loading and release. As there have been few studies on this, our study is one of the first to examine this aspect of such sponges.
Here, the sponges were cut into uniform pieces of 1×1 × 0.5 cm. Seven different concentrations of gentamicin solution were used, and we chose five time points at which to measure the air-dried sponges. The amount of gentamicin absorbed by the sponges increased gradually over 96 h. As the sponges began to obviously decompose in water at about 96 h, their drug loading significantly decreased in the 4, 1.6, and 0.8 mg/mL air-dried sponge groups after 96 h; thus, gentamicin sponges’ immersion time in water should not exceed 96 h. We constructed the gentamicin loading equation through in vitro experiments and tested its fit, which was over 0.9, meaning that its accuracy was very high and could provide a reference for future study of sponge drug loading.
In terms of the in vitro drug release experiment on the gentamicin sponges, we primarily considered whether the amount of gentamicin released from the sponges could reach the therapeutic concentration. The effective treatment concentration of gentamicin is 4–10 μg/mL.20,21 In the 4, 1.6, and 0.8 mg/mL air-dried sponge groups, the amount of gentamicin released did not reach the effective therapeutic concentration at two hours but did so at six hours. That is, the air-dried gentamicin-saturated sponges immersed in 4, 1.6, and 0.8 mg/mL of the gentamicin solution could not exert gentamicin’s bactericidal ability within two hours. However, the amount of gentamicin released from the 40 and 16 mg/mL air-dried sponges was 16.3 and 4.7 μg/mL, respectively, so these two sponges were able to exert the bactericidal ability at 30 min. We constructed the release equation for the gentamicin sponges in vitro to quantify their drug release. The equation’s fit was analyzed, and its R2 was over 0.9, meaning that its accuracy was high. Thus, the equation could be used to analyze the bactericidal ability of gentamicin sponges.
As the amount of gentamicin released by some sponges was lower than the therapeutic concentration within two hours, we used bacterial and animal experiments to analyze whether the different groups of gentamicin sponges had a similar bactericidal effect. Although the bactericidal areas of the 40 and 16 mg/mL air-dried sponge groups were significantly larger than those of the other groups, the 1.6 and 0.8 mg/mL air-dried sponge groups also had obvious bactericidal effects (compared with the 0 mg/mL air-dried sponge group). Based on this, we considered that the 1.6 and 0.8 mg/mL air-dried sponges were sufficient to prevent wound infection.
For the animal experiments, we established a rat femur fracture model combined with MSSA or P. aeruginosa wound infections. The model’s stability was analyzed by the animal mortality and wound suppuration rates. Finally, we chose 108 CFU/mL of MSSA and 106 CFU/mL of P. aeruginosa to establish the rat model. Using different gentamicin sponges, we found that the 40, 16, and 8 mg/mL air-dried sponges could reduce the wound infection rate to zero. Therefore, if a wound has been confirmed to be infected and the bacteria have been identified as sensitive, we recommend that a 40, 16, or 8 mg/mL air-dried sponge be used to treat the infection.
The innovation of this study is that the drug loading and release of gentamicin sponges have been quantified for the first time and that an equation with a fit of over 0.9 has been constructed. Second, we have proposed which gentamicin sponges should be used to prevent and treat wound infections. Third, we have provided a basis for future research on wound infection models and gentamicin sponges.
There are also many limitations to this study. First, the sponges we used were produced by the same factory, so the results may be different when using sponges produced by other manufacturers in in vitro drug loading and release experiments. Second, we chose MSSA and P. aeruginosa to conduct the bacteriostatic tests in vitro and on the infected rats, so the results cannot represent other bacteria, eg, Escherichia coli or Staphylococcus epidermidis. Third, the incidence of wound infection in rodents is significantly different than that in humans, so further studies are required to analyze which gentamicin sponges are sufficient for the prevention or treatment of wound infections in humans (Figure 6).
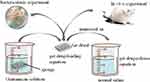 |
Figure 6 The purpose of this study was to investigate the effects of gentamicin sponges in vitro and in vivo. |
Conclusion
In this study, we constructed drug-loading and drug-release equations for gentamicin sponges for the first time, and the equations’ fit was over 0.9. The equations could therefore be used to analyze the gentamicin sponges further. Moreover, we proposed that 1.6 and 0.8 mg/mL air-dried sponges are sufficient to prevent wound infections; in addition, 40, 16, and 8 mg/mL air-dried sponges can be used to treat wound infections. We recommend that sponges not be soaked in gentamicin for more than 96 h due to decomposition.
Abbreviations
RCTs, randomized controlled trials; SSI, Surgical site infection; LB, Luria Broth; PBS, phosphate buffer saline; MSSA, Methicillin sensitive Staphylococcus aureus; P. aeruginosa, Pseudomonas aeruginosa; SD, Sprague-Dawley.
Data Sharing Statement
The supplemental data used to support the findings of this study have been deposited in the Mendeley Data repository (DOI:10.17632/vpj7vjkz86.1).
Ethical Approval and Consent to Participate
This article does not contain any studies with human participants performed by any of the authors. The present study was approved by the Capital Medical University Ethics Committee on the use of animals in research and education.
Consent for Publication
All authors agree for publication.
Acknowledgments
We thank that medical research center of Beijing Chaoyang Hospital affiliated to Capital Medical University provides the experimental place.
Funding
This work is Supported by Beijing Natural Science Foundation (7202049) which was obtained by Junlin Zhou. The role of the funding was in experiments and collection.
Disclosure
The authors have no conflicts of interest or financial disclosures.
References
1. Bennett-Guerrero E, Pappas TN, Koltun WA, et al. Gentamicin–collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med. 2010;363(11):1038–1049. doi:10.1056/nejmoa1000837
2. Marson BA, Grindlay DJC, Ollivere BJ, Deshmukh SR, Scammell BE. A systematic review of local antibiotic devices used to improve wound healing following the surgical management of foot infections in diabetics. Bone Jt J. 2018;100-B(11):1409–1415. doi:10.1302/0301-620X.100B11.BJJ-2018-0720
3. Bennett-Guerrero E, Ferguson TB, Lin M, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA J Am Med Assoc. 2010. doi:10.1001/jama.2010.1152
4. Han J-S, Kim S-H, Jin S-W, et al. The use of gentamicin-impregnated collagen sponge for reducing surgical site infection after spine surgery. Korean J Spine. 2016;13(3):129. doi:10.14245/kjs.2016.13.3.129
5. Chang WK, Srinivasa S, MacCormick AD, Hill AG. Gentamicin-collagen implants to reduce surgical site infection: systematic review and meta-analysis of randomized trials. Ann Surg. 2013;258(1):59–65. doi:10.1097/SLA.0b013e3182895b8c
6. Schimmer C, Özkur M, Sinha B, et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: a controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg. 2012;143(1):194–200. doi:10.1016/j.jtcvs.2011.05.035
7. Wouthuyzen-Bakker M, Löwik CAM, Knobben BAS, et al. Use of gentamicin-impregnated beads or sponges in the treatment of early acute periprosthetic joint infection: a propensity score analysis. J Antimicrob Chemother. 2018. doi:10.1093/jac/dky354
8. Uçkay I, Kressmann B, Di Tommaso S, et al. A randomized controlled trial of the safety and efficacy of a topical gentamicin–collagen sponge in diabetic patients with a mild foot ulcer infection. SAGE Open Med. 2018;6:205031211877395. doi:10.1177/2050312118773950
9. Husodo K, Kamal AF, Yusuf AA. Effect of povidone iodine and hydrogen peroxide on fracture healing: a histomorphometric study on rats. J Orthop Surg. 2016;24(2):245–249. doi:10.1177/1602400224
10. Wang D, Liu Y, Zhao YR, Zhou JL. Low dose of lipopolysaccharide pretreatment can alleviate the inflammatory response in wound infection mouse model. Chinese J Traumatol. 2016;19(4):193–198. doi:10.1016/j.cjtee.2016.06.001
11. Kowalewski M, Pawliszak W, Zaborowska K, et al. Gentamicin-collagen sponge reduces the risk of sternal wound infections after heart surgery: meta-analysis. J Thorac Cardiovasc Surg. 2015;149(6):1631–1640.e6. doi:10.1016/j.jtcvs.2015.01.034
12. Rapetto F, Bruno VD, Guida G, Marsico R, Chivasso P, Zebele C. Gentamicin-impregnated collagen sponge: effectiveness in preventing sternal wound infection in high-risk cardiac surgery. Drug Target Insights. 2016;10s1:
13. Birgand G, Radu C, Alkhoder S, et al. Does a gentamicin-impregnated collagen sponge reduce sternal wound infections in high-risk cardiac surgery patients? Interact Cardiovasc Thorac Surg. 2013;16(2):134–141. doi:10.1093/icvts/ivs449
14. Schimmer C, Gross J, Ramm E, et al. Prevention of surgical site sternal infections in cardiac surgery: a two-centre prospective randomized controlled study. Eur J Cardiothorac Surg. 2017;51(1):67–72. doi:10.1093/ejcts/ezw225
15. Hayes G, Moens N, Gibson T. A review of local antibiotic implants and applications to veterinary orthopaedic surgery. Vet Comp Orthop Traumatol. 2013. doi:10.3415/VCOT-12-05-0065
16. Pochhammer J, Zacheja S, Schäffer M. Subcutaneous application of gentamicin collagen implants as prophylaxis of surgical site infections in laparoscopic colorectal surgery: a randomized, double-blinded, three-arm trial. Langenbecks Arch Surg. 2015;400(1):1–8. doi:10.1007/s00423-014-1232-4
17. Formanek MB, Herwaldt LA, Perencevich EN, Schweizer ML. Gentamicin/collagen sponge use may reduce the risk of surgical site infections for patients undergoing cardiac operations: a meta-analysis. Surg Infect (Larchmt). 2014;15(3):244–255. doi:10.1089/sur.2012.209
18. Godbole G, Pai V, Kolvekar S, Wilson APR. Use of gentamicin-collagen sponges in closure of sternal wounds in cardiothoracic surgery to reduce wound infections. Interact Cardiovasc Thorac Surg. 2012;14(4):390–394. doi:10.1093/icvts/ivr129
19. Migaczewski M, Zub-Pokrowiecka A, Budzyński P, Matłok M, Budzyński A. Prevention of early infective complications after laparoscopic splenectomy with the Garamycin sponge. Wideochir Inne Tech Maloinwazyjne. 2012. doi:10.5114/wiitm.2011.27151
20. Andersson DI, Hughes D. Selection and transmission of antibiotic-resistant bacteria. Microb Transm. 2017. doi:10.1128/microbiolspec.mtbp-0013-2016
21. Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi:10.1038/nrmicro3380
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


