Back to Journals » Journal of Inflammation Research » Volume 15
A Potential Role of B7-H4 Expression in Predicting the Recurrence of Chronic Rhinosinusitis with Nasal Polyps
Authors Wang F, Chu W, Deng Z, Jing Q, Xie B
Received 16 February 2022
Accepted for publication 2 June 2022
Published 9 June 2022 Volume 2022:15 Pages 3421—3431
DOI https://doi.org/10.2147/JIR.S361868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Fengjun Wang,1,2 Wei Chu,3 Zhenghao Deng,2,4 Qiancheng Jing,5 Bin Xie2,4
1Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China; 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital of Central South University, Changsha, Hunan, 410008, People’s Republic of China; 3Department of Pathology, The People’s Hospital of Shimen County, Changde, Hunan, People’s Republic of China; 4Department of Pathology, Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China; 5The Affiliated Changsha Central Hospital, Department of Otolaryngology Head and Neck Surgery, Hengyang Medical School, University of South China, Changsha, Hunan, People’s Republic of China
Correspondence: Bin Xie, Department of Pathology, Xiangya Hospital of Central South University, No. 87 Xiangya Road, Kaifu District, Changsha, Hunan Province, People’s Republic of China, Email [email protected]
Background: Chronic rhinosinusitis with polyps (CRSwNP) is a common chronic inflammatory disease of the nasal cavity and sinuses with a high rate of postoperative recurrence. In this study, we aim to investigate the expression of B7-H4 in CRSwNP and its association with postoperative recurrence.
Methods: A total of 80 CRSwNP patients, including 40 primary CRSwNP (pCRSwNP) patients and 40 recurrent CRSwNP (rCRSwNP) patients, 27 chronic rhinosinusitis without polyps (CRSsNP) and 32 healthy controls (HC) were enrolled in this study, and the serum, nasal polyps and middle turbinate tissue samples were collected. Peripheral and tissue B7-H4 expressions were detected by enzyme-linked immunosorbent assay (ELISA) and reverse transcription-polymerase chain reaction (RT-PCR) and immunofluorescence, and their clinical values in predicting postoperative recurrence of CRSwNP were evaluated.
Results: We identified significantly higher tissue B7-H4 mRNA levels in the CRSwNP group than in the HC group, and elevated B7-H4 levels were associated with tissue eosinophil count and percentage (r = 0.469, P < 0.001; r = 0.521, P < 0.001). B7-H4 mRNA and protein levels were significantly higher in the rCRSwNP group than the pCRSwP group. Multivariate analysis and receiver operating characteristic (ROC) curves showed that tissue B7-H4 levels were associated with postoperative recurrence in patients with CRSwNP (P < 0.05). In addition, serum B7-H4 levels were significantly increased in the CRSwNP group than the CRS and HC groups, especially in the rCRSwNP group (P < 0.05), and the ROC curve presented a predictive ability of serum B7-H4 in predicting postoperative recurrence.
Conclusion: Our results indicated that B7-H4 level was clearly enhanced in CRSwNP patients and associated with postoperative recurrence. Serum B7-H4 might serve as a simple and convenient biomarker for early predicting postoperative recurrence in CRwNP patients.
Keywords: B7-H4, chronic rhinosinusitis with nasal polyps, recurrence, biomarker
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous disease of the upper respiratory tract with chronic inflammation in nasal and paranasal sinuses mucosa persisting for at least 12 weeks.1 The common symptoms of CRS include nasal obstruction, drainage, smell loss, and facial pain or pressure.2 CRS is frequently further divided into two subtypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP) based on the presence of nasal polyps.3–6 Previous studies have shown that CRSwNP was characterized by T helper 2 (Th2)-dominant inflammation and tissue eosinophils infiltration, which resulted in serious disease symptoms, poorer prognosis, and high recurrence rate of postoperative.7,8 Recently, macrophages have been observed in nasal polyps, and the accumulation of mannose-receptor positive macrophages (M2 macrophages) was identified in nasal polyps in cell aggregates which suggests that they play a key role in the pathogen-macrophage interaction in CRSwNP.9 Although medical treatment and nasal endoscopic surgery were the mainstream treatment in CRSwNP, there remains a large number of patients still suffer from relapse. Meng et al followed up 230 CRSwNP patients for more than 24 months after surgery, and they found that 51.3% of them were recurrent.10 Moreover, other publications reported that the short-term recurrence rates of CRSwNP ranged from 21% to 66%.11 Considering that the disease has a higher rate of recurrence, there is an urgent need to explore biomarkers for early prediction of nasal polyp recurrence, which may contribute to developing treatment strategies, adjusting follow-up protocols, and achieving precision treatment.
B7-H4, also known as B7S1, B7x, VTCN1, is widely expressed on most antigen-presenting cells (APCs) including dendritic cells, peritoneal macrophages, and splenic B cells.12,13 Moreover, B7-H4 is also the cell surface marker of M2 macrophages and crucial in maintaining the function of macrophages.14 Previous studies have shown that B7-H4 acts an essential role in tumor, organ transplantation rejection, inflammatory and autoimmune diseases.14–18 A recent publication found that serum B7-H4 levels were elevated with disease progression in allergic and autoimmune diseases mediated by T helper 1 (Th1) or Th2 cell activation.19 In addition, previous studies found that B7 promoted IL-10 production, which could enhances the migration of eosinophils.20,21 Given that, we speculate that B7-H4 may be involved in the pathophysiology of CRSwNP and contribute to its postoperative recurrence. Therefore, the present study aims to explore the role of B7-H4 in CRSwNP and clinical value as an objective marker to predict postoperative recurrence via detecting its tissue and serum expression levels in CRSwNP patients.
Materials and Methods
Participants and Settings
We recruited 32 health controls (HC) and 27 CRSsNP patients, and 80 CRSwNP patients including 40 primary CRSwNP (pCRSwNP) and 40 recurrent CRSwNP (rCRSwNP) who were treated in our hospital between October 2020 and June 2021. Diagnosis of CRSwNP and CRSsNP was strictly confirmed according to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS).22 Recurrent CRSwNP is defined as: despite the rescue plan of antibiotics and oral steroids during follow-up, there are still typical symptoms, endoscopic images and/or computed tomography (CT) scan evidence were obtained during outpatient clinic follow-up as previously described.10,23,24 None of the patients were treated with glucocorticoids, immunomodulatory agents, or antibiotics within 4 weeks before enrollment. Patients with fungal sinusitis, allergic fungal rhinosinusitis, cystic fibrosis, or primary ciliary dyskinesia were excluded. Age and sex-matched health controls without nasal or sinus inflammatory diseases were similarly recruited as control group. This study was approved by the Institutional Review Board of Xiangya Hospital. Informed consent was obtained from the patients enrolled.
RNA Extraction, Reverse Transcription and Quantitative Realtime Polymerase Chain Reaction (qRT-PCR) Amplification
Middle turbinate mucosa for CRSsNP patients and HCs, and nasal polyp specimens from CRSwNP patients were collected during functional endoscopic sinus surgery and immediately snap-frozen in liquid nitrogen. Total RNA in tissue sample was extracted by the Trizol® method following the manufacturer’s instructions. The quality of total RNA was assessed with the Nanodrop-2000 (Thermo Fisher Scientific, Waltham, MA, USA). Single-strand cDNA was synthesized with PrimeScript™ RT Master Mix (US EVERBRIGHT, Suzhou, China), and aliquots of cDNA equivalent to 10 ng of total RNA in each well were used for qRT-PCR. The qRT-PCR was performed as described previously.25 Briefly, qRT-PCR was performed using 10 ng of cDNA with the SYBR Green qPCR Supermix (US EVERBRIGHT, Suzhou, China) to monitor DNA synthesis using specific primers. The mRNA expression of gene was calculated using the comparative threshold cycle (2−ΔΔCT) method. GAPDH was used as the control and the primers used for amplifying GAPDH and B7-H4 were displayed in Table S1.
Immunofluorescence Staining
Collected tissue specimens were fixed 4% paraformaldehyde, embedded with paraffin, sectioned into 5-μm sections by microtome. The tissue sections were first stained with primary antibody to B7-H4 (Affinity Biosciences, Changzhou, China) overnight at 4°C. After washing, the tissue sections were incubated with Alexa Fluor 594–conjugated secondary antibodies at room temperature for 2 hours.26 The numbers of positive cells of each type in the lamina propria per high-power field (HPF) were counted, as previously described.27 Histological changes and B7-H4 expressions in the sections were observed by two independent pathologists and the presentative images were selected and displayed in the Results Section.
Enzyme-Linked Immunosorbent Assay (ELISA)
In order to detect the serum B7-H4 concentrations in all subjects, we conducted ELISA.
Five mL fasting blood was collected from each participant with vacuum blood collection tubes. Harvested samples were coagulated at room temperature for 1 h and centrifuged at 3000 rpm for 10 min at 4°C, then, the supernatant was collected and stored for subsequent test. Serum B7-H4 levels were quantified by ELISA kit (CUSABIO, Wuhan, China) referring to the manufacturer’s instructions. Operator was blinded to the clinical data of patients.
Statistical Analysis
All data were expressed as the mean± standard deviation. One-way analysis of variance (ANOVA) or Student’s t-test was conducted when the variables distributed normally, otherwise, Kruskal–Wallis H-test or Mann–Whitney U-test was performed. Correlations between mRNA level of B7-H4 and clinical variables were assessed by Spearman’s rank correlation analysis. Multivariate analysis and receiver operating characteristic (ROC) curves were performed to identify predictive factors associated with polyp recurrence and determine the predictive values of special parameters, based on the area under the curves (AUCs) for a particular characteristic. Statistical analyses were performed with SPSS statistics software version 25.0 (IBM, Chicago, IL, USA). A P value < 0.05 was defined as statistically significant.
Results
Demographics of the Study Subjects
Demographic and clinical data are shown in Table 1. Results indicated that the rate of allergic rhinitis, peripheral eosinophil count and percentage were significantly increased in CRSwNP group than the other two groups (all P < 0.05). The VAS score and Lund-Mackay score were higher in CRSwNP group than CRSsNP group (all P < 0.001). No significant difference was found in sex, age, BMI among three groups. As shown in Table 2, the patients in the recurrent group had significantly higher rate of allergic rhinitis, peripheral eosinophil count and percentage, and VAS score than in the primary group (all P < 0.05), and no statistical difference was observed between two groups for the other variables.
 |
Table 1 The Demographic and Clinical Characteristics Among Three Groups |
 |
Table 2 The Demographic and Clinical Parameters Between pCRSwNP Group and rCRSwNP |
B7-H4 Expression Level in Nasal Tissues and Its Correlation with Clinical Variables
As exhibited in Figure 1A, the average tissue B7-H4 mRNA expression levels were significantly higher in the CRSwNP group than CRSsNP group and HC group, but no statistic difference was observed between CRSsNP group and HC group. CRSwNP group were further divided into CRSwNP with allergic rhinitis (AR) group and CRSwNP without AR group, and CRSwNP with asthma (AS) group and CRSwNP without AS group based on the comorbidity. The expression levels of B7-H4 were not statistically different between these subgroups (Figure 1B and C). The Spearman correlation results in Figure 2 showed that tissue B7-H4 levels were positively correlated with tissue eosinophil count (r = 0.469, P < 0.001) and tissue eosinophil percentage (r = 0.521, P < 0.001), respectively. The relative B7-H4 mRNA levels were significantly higher in tissue from rCRSwNP patients than pCRSwNP patients (P < 0.001, Figure 3A). Logistic regression analysis results demonstrated that tissue B7-H4 mRNA levels were associated with CRSwNP postoperative recurrence (Table 3). ROC curve in Figure 3B showed that tissue B7-H4 mRNA level presented a potential ability in predicting CRSwNP postoperative recurrence (AUC = 0.788, P < 0.001).
 |
Table 3 Logistic Regression Analysis of Factors Associated with the Rate of Recurrence in CRSwNP Patients |
Immunofluorescence Staining for B7-H4
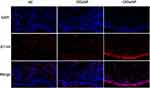
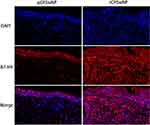
As indicated by representative immunofluorescence staining images (Figure 3), the B7-H4 protein expression level was significantly elevated in the CRSwNP group in comparison with HC and CRSsNP groups, and markedly enhanced in the rCRSwNP group than pCRSwNP group (Figure 4). B7-H4 staining was mainly located in the nasal epithelial cells and submucosa areas, and the number of B7-H4+ positive cells was increased in CRSwNP group in comparison with HC and CRSsNP groups (Figure 5), and increased number of B7-H4+ positive cells was also found in recurrent group than primary group (Figure 6).
Serum B7-H4 Concentrations in CRSwNP
Serological results highlighted that B7-H4 concentrations were significantly elevated in the CRSwNP group than CRSsNP and HC group, and the serum B7-H4 concentrations were significantly enhanced in the rCRSwNP group than pCRSwNP group (P < 0.05, Figure 7A and B). Binary logistic regression analysis results in Table 3 demonstrates that serum B7-H4 level (OR = 6.837, P < 0.001) was associated with CRSwNP postoperative recurrence. ROC curves showed that serum B7-H4 (AUC = 0.852) presented potential value in predicting postoperative recurrence (Figure 7C). Intriguingly, the Spearman correlation results showed that serum B7-H4 concentrations positively correlated with the B7-H4 mRNA expression levels in nasal polyps in CRSwNP patients (r = 0.470, P < 0.001) (Figure S1).
Discussion
In the present study, our results showed that tissue B7-H4 levels were significantly enhanced in the CRSwNP group, especially in the rCRSwNP patients, and elevated B7-H4 levels were positively with the degree of tissue eosinophil infiltration. Statistical analysis results showed that tissue B7-H4 levels were closely linked with CRSwNP recurrence. Moreover, serological results were consistent with the above findings, and binary logistic regression and ROC curve analysis demonstrated that circulating B7-H4 levels were associated with CRSwNP recurrence. Based on these results, we believe that B7-H4 may be involved in the pathogenesis of CRSwNP, and promote its recurrence after surgery. Therefore, tissue and serum B7-H4 level may serve as potential biomarkers for predicting postoperative recurrence in CRwNP patients.
Macrophages are important members of innate immune cells which participate in adaptive immunity,28 and their phenotype and functions are affected by the surrounding micro-environment.29 Previous studies have shown that classically activated (M1) and alternatively activated (M2) macrophages are two major macrophage sub-populations, and they exhibited different biological functions and are involved in the occurrence and development of various diseases.30,31 Accordingly, M1 macrophages are typically induced by Th1 cytokines, such as IFN-γ and TNF-α, while M2 macrophages are polarized by Th2 cytokines and exhibit anti-inflammatory effects.32–35 It has been proven that CRSwNP is characterized by Th2-related inflammation, and M2 macrophages serve crucial roles in promoting Th2 inflammation response and are closely involved in pathological mechanisms of CRSwNP. Recent studies observed that the number of M2 macrophages but not M1 macrophages were significantly increased in the peripheral blood and tissue of CRSwNP patients.36,37 B7-H4, as an important cell surface marker of M2 macrophages, may be involved in the occurrence and development of chronic inflammatory diseases, including CRSwNP. Xiao et al38 found that the concentration of soluble B7-H4 was significantly higher in patients with systemic lupus erythematosus and lupus nephritis in comparison with healthy subjects, and the elevated B7-H4 levels correlated with the disease severity. In another study, Chen et al39 found that B7-H4 was expressed in CD34(+) endothelial cells of neovessels in rheumatoid synovium, which suggested that B7-H4 was involved in the pathological changes of rheumatoid synovium in rheumatoid arthritis. In this study, we observed that tissue B7-H4 mRNA and protein expression levels were elevated in the CRSwNP group in comparison with the HC and CRSsNP groups, and B7-H4 mRNA levels were associated with tissue eosinophilia. Prior studies found that tissue eosinophil infiltration plays an important role in the occurrence and progression of CRSwNP, and M2 macrophages could promote eosinophil migration via secreting chemokines, such as C-C motif chemokine ligand 17 (CCL17), CCL18, CCL22, and CCL24.40,41 Therefore, we speculated that B7-H4 might activate M2 macrophages, then triggered Th2 inflammatory response and eosinophils recruitment, resulting in excessively inflammatory conditions in CRSwNP.
Currently, although functional endoscopic sinus surgery and pharmaceutical treatment improve the quality of life in CRSwNP patients, a large portion of patients still suffered a high rate of recurrence, especially in those with tissue eosinophilia. Therefore, exploring risk factors associated with CRSwNP recurrence and predicting its early recurrence are urgently needed for rhinologists. Previous publications showed that tissue and peripheral eosinophil count and percentage were associated with the relapse of CRSwNP, but their predictive abilities for postoperative recurrence needed further validation.42,43 In this study, we firstly demonstrated that soluble and membrane-bound forms of B7-H4 expression levels were significantly elevated in rCRSwNP patients, and were closely linked with the rate of CRSwNP postoperative recurrence. Moreover, binary logistic regression and ROC curve analyses demonstrated that both forms of B7-H4 presented promising values for predicting CRSwNP recurrence. Growing evidence suggested that Th2 inflammation and eosinophilic inflammation were vital endotypes associated with poorly-controlled disease and recurrence in patients with CRSwNP.42,44 A recent study showed that serum B7-H4 levels were elevated with disease progression in allergic and autoimmune diseases.19 Another prior study found that serum B7-H4 levels were enhanced in the ovalbumin (OVA) induced animal models and associated with the concentrations of Th2 cytokines, which suggested that B7-H4 might be a key promoter for allergic inflammation.19 In the present study, our results highlighted that tissue eosinophil count and percentage were raised in CRSwNP patients and were related with the postoperative recurrence, which was in accordance with prior studies. Besides, tissue and serum B7-H4 expression levels were clearly elevated in the rCRSwNP group than the pCRSwNP group, and the tissue B7-H4 levels were positively correlated with tissue eosinophil count and percentage, which suggested that B7-H4 might facilitate eosinophilia and be emerged in the recurrence CRSwNP. All above findings suggested that serum and tissue B7-H4 might serve as potential clinical biomarkers for preoperatively predicting CRSwNP recurrence.
There are several limitations in our study. First, the sample size of participants included in the study was relatively small, making the statistical analysis challenging. Second, we did not conduct a validation cohort to strengthen the results in this study. Finally, we did not analyze the correlation between the B7-H4 expression level and the severity of the disease. Further multicenter studies with a larger sample size are needed to confirm the results of this study and explore the accuracy mechanisms of B7-H4 in CRSwNP.
In conclusion, we confirmed the association between B7-H4 and CRSwNP in the present study. We firstly demonstrated that both soluble and membrane-bound forms of B7-H4 were overexpressed in CRSwNP and associated with postoperative recurrence. Serum B7-H4 might serve as a simple and convenient biomarker for early predicting postoperative recurrence in CRSwNP patients.
Data Sharing Statement
The data used to support the observations of this study are available from the corresponding author upon request.
Ethics Statement
This study was conducted in accordance with the recommendations of Declaration of Helsinki. The Human Ethical Committee of Xiangya Hospital of Central South University approved this study, all participants provided informed consent.
Funding
This work was supported by the Natural Science Foundation of Hunan Province (No.2020JJ4910) and Changsha Municipal Natural Science Foundation (kq2007050).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128(6):1198–1206. doi:10.1016/j.jaci.2011.08.037
2. Hulse KE, Stevens WW, Tan BK, et al. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45(2):328–346. doi:10.1111/cea.12472
3. Staudacher AG, Peters AT, Kato A, et al. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;124(4):318–325. doi:10.1016/j.anai.2020.01.013
4. Cho SH, Hamilos DL, Han DH, et al. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1505–1511. doi:10.1016/j.jaip.2019.12.021
5. McCormick JP, Thompson HM, Cho DY, et al. Phenotypes in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2020;20(7):20. doi:10.1007/s11882-020-00916-6
6. Grayson JW, Cavada M, Harvey RJ. Clinically relevant phenotypes in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2019;48(1):23. doi:10.1186/s40463-019-0350-y
7. Bailey LN, Garcia J, Grayson JW. Chronic rhinosinusitis: phenotypes and endotypes. Curr Opin Allergy Clin Immunol. 2021;21(1):24–29. doi:10.1097/ACI.0000000000000702
8. Ma J, Shi LL, Deng YK, et al. CD8(+) T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2016;46(9):1162–1175. doi:10.1111/cea.12758
9. Claeys S, De Belder T, Holtappels G, et al. Macrophage mannose receptor in chronic sinus disease. Allergy. 2004;59(6):606–612. doi:10.1111/j.1398-9995.2004.00471.x
10. Meng Y, Zhang L, Lou H, et al. Predictive value of computed tomography in the recurrence of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2019;9(11):1236–1243. doi:10.1002/alr.22355
11. Morrissey DK, Bassiouni A, Psaltis AJ, et al. Outcomes of modified endoscopic Lothrop in aspirin-exacerbated respiratory disease with nasal polyposis. Int Forum Allergy Rhinol. 2016;6(8):820–825. doi:10.1002/alr.21739
12. Sica GL, Choi IH, Zhu G, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi:10.1016/S1074-7613(03)00152-3
13. Prasad DV, Richards S, Mai XM, et al. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18(6):863–873. doi:10.1016/S1074-7613(03)00147-X
14. Abumaree MH, Al JM, Kalionis B, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. 2013;9(5):620–641. doi:10.1007/s12015-013-9455-2
15. Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6(8):759–766. doi:10.1016/j.micinf.2004.03.007
16. Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6(6):223. doi:10.1186/gb-2005-6-6-223
17. Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34(11):556–563. doi:10.1016/j.it.2013.07.003
18. Guo G, Shang Y, Zhu G, et al. The expression and distribution of immunomodulatory proteins B7-H1, B7-DC, B7-H3, and B7-H4 in rheumatoid synovium. Clin Rheumatol. 2012;31(2):271–281. doi:10.1007/s10067-011-1815-1
19. Kamimura Y, Kobori H, Piao J, et al. Possible involvement of soluble B7-H4 in T cell-mediated inflammatory immune responses. Biochem Biophys Res Commun. 2009;389(2):349–353. doi:10.1016/j.bbrc.2009.08.144
20. Makita N, Hizukuri Y, Yamashiro K, et al. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol. 2015;27(3):131–141. doi:10.1093/intimm/dxu090
21. Wang X, Wang T, Xu M, et al. B7-H4 overexpression impairs the immune response of T cells in human cervical carcinomas. Hum Immunol. 2014;75(12):1203–1209. doi:10.1016/j.humimm.2014.10.002
22. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi:10.4193/Rhin20.401
23. Rosati D, Rosato C, Pagliuca G, et al. Predictive markers of long-term recurrence in chronic rhinosinusitis with nasal polyps. Am J Otolaryngol. 2020;41(1):102286. doi:10.1016/j.amjoto.2019.102286
24. Ninomiya T, Noguchi E, Haruna T, et al. Periostin as a novel biomarker for postoperative recurrence of chronic rhinosinitis with nasal polyps. Sci Rep. 2018;8(1):11450. doi:10.1038/s41598-018-29612-2
25. Yan B, Wang Y, Li Y, et al. Inhibition of arachidonate 15-lipoxygenase reduces the epithelial-mesenchymal transition in eosinophilic chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2019;9(3):270–280. doi:10.1002/alr.22243
26. Yao Y, Wang ZC, Liu JX, et al. Increased expression of TIPE2 in alternatively activated macrophages is associated with eosinophilic inflammation and disease severity in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2017;7(10):963–972. doi:10.1002/alr.21984
27. Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(3):
28. Zhang M, Liu F, Zhou P, et al. The MTOR signaling pathway regulates macrophage differentiation from mouse myeloid progenitors by inhibiting autophagy. Autophagy. 2019;15(7):1150–1162. doi:10.1080/15548627.2019.1578040
29. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
30. Sica A, Erreni M, Allavena P, et al. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. doi:10.1007/s00018-015-1995-y
31. Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–566. doi:10.1146/annurev-physiol-022516-034339
32. Bashir S, Sharma Y, Elahi A, et al. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res. 2016;65(1):1–11. doi:10.1007/s00011-015-0874-1
33. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi:10.1016/j.immuni.2014.06.008
34. Porta C, Riboldi E, Ippolito A, et al. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27(4):237–248. doi:10.1016/j.smim.2015.10.003
35. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi:10.3389/fimmu.2014.00614
36. Krysko O, Holtappels G, Zhang N, et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy. 2011;66(3):396–403. doi:10.1111/j.1398-9995.2010.02498.x
37. Kadoch C, Dinca EB, Voicu R, et al. Pathologic correlates of primary central nervous system lymphoma defined in an orthotopic xenograft model. Clin Cancer Res. 2009;15(6):1989–1997. doi:10.1158/1078-0432.CCR-08-2054
38. Xiao JP, Wang XR, Zhang S, et al. Increased serum levels of soluble B7-H4 in patients with systemic lupus erythematosus. Iran J Immunol. 2019;16(1):43–52. doi:10.22034/IJI.2019.39405
39. Chen L, Lu Y, Wang F, et al. Expression of co-stimulatory molecule B7-H4 in patients suffering from rheumatoid arthritis. Immunol Lett. 2013;154(1–2):25–30. doi:10.1016/j.imlet.2013.07.009
40. Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121(6):
41. Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi:10.1016/j.it.2004.09.015
42. Brescia G, Contro G, Giacomelli L, et al. Blood eosinophilic and basophilic trends in recurring and non-recurring eosinophilic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2021;35(3):296–301. doi:10.1177/1945892420953960
43. Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70(8):995–1003. doi:10.1111/all.12644
44. Ho J, Li W, Grayson JW, et al. Systemic medication requirement in post-surgical patients with eosinophilic chronic rhinosinusitis. Rhinology. 2021;59(1):59–65. doi:10.4193/Rhin20.073
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.