Back to Journals » International Journal of Nanomedicine » Volume 15
A Potent and Safer Anticancer and Antibacterial Taxus-Based Green Synthesized Silver Nanoparticle
Authors Sarli S, Kalani MR , Moradi A
Received 25 February 2020
Accepted for publication 13 May 2020
Published 28 May 2020 Volume 2020:15 Pages 3791—3801
DOI https://doi.org/10.2147/IJN.S251174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Sona Sarli,1 Mohamad Reza Kalani,2,* Abdolvahab Moradi3,*
1Department of Chemistry, Arak Islamic Azad University, Arak, Iran; 2Medical Cellular and Molecular Research Center, School of Advanced Medical Technologies, Golestan University of Medical Sciences, Gorgan, Iran; 3Department of Microbiology, College of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
*These authors contributed equally to this work
Correspondence: Abdolvahab Moradi; Mohamad Reza Kalani Email [email protected]; [email protected]
Purpose: Paclitaxel is a generic drug produced based on Taxol which is an extract of Taxus tree, well known for its anticancer and antibacterial effects. This study was aimed at building up an agent with the antibacterial and anticancer benefits of both the silver ions and Taxol, together with less cytotoxic effects.
Materials and Methods: Colloidal silver nanoparticles (AgNPs) were synthesized by reducing aqueous AgNO3 with aqueous Taxus leaf extract at nonphotomediated conditions, without any catalyst, template or surfactant. The AgNP production was confirmed by ultraviolet-visible (UV-VIS) spectroscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier-transform infrared (FTI) spectroscopy. The MTT assay for human breast cancer cells as well as the DAPI fluorescent staining microscopy tested the biocompatibility and anticancer effects of AgNPs, silver nitrate, and Taxol. Transmission electron microscopy (TEM) and dynamic light scattering (DLS) techniques were performed to determine the shape and size of the nanoparticles. MTT assay showed the best inhibitory concentration of AgNPs on cancer cells. The antibacterial activity of the three case study materials was tested for gram-positive (Staphylococcus aureus) and gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) using well diffusion test.
Results: This work proposes more anticancer effects for AgNP made by Taxus brevifolia extract, comparing Taxol solution. IC50 was observed as 3.1 mM for Taxol while 1.5 mM for new AgNP. Moreover, Taxus showed no antibacterial effects while the new AgNP showed a dose-dependent biocompatibility along with slightly more antibacterial effects (MIC: 1.6 and 6.6mM for gram-positive and -negative bacteria, respectively) comparing with silver nitrate solution (MIC: 1.5 and 6.2 mM for gram-positive and -negative bacteria, respectively).
Conclusion: The production of herbal-mediated silver nanoparticles may be an efficient substitution for the silver nitrate–based medicines with less side effects.
Keywords: Taxus tree extract, green synthesis, silver nanoparticle, antibacterial, anticancer
Introduction
Over the past few decades, researchers have paid attention to plant extracts as the treatment for various diseases, such as some bacterial infections and cancer.1–6 Taxol is an extract of the Taxus tree (Scientific name: Taxus brevifolia, Family: Taxaceae), which has been shown to have anticancer effects.4,6,7 The American National Cancer Institute (NCI) began the first studies of the antimicrobial properties of Taxol in the late 1950s which is still a popular research topic.8,9 Taxol has been shown to have suppressive effects on a variety of breast, skin and ovarian cancers by preventing the de-polymerization of tubulins. A generic drug named “paclitaxel” has produced based on Taxol which has a registered trade name as Taxol(R) BMS [Bristol-Myers Squibb].
The other aspect of the current study was the synthesis of silver nanoparticles (AgNP). Recently, phyto-nanotechnology has proposed a new “green” method for the synthesis of nanoparticles which are eco-friendly, stable, rapid, simple, and cost-effective.10,11 Although the “Green synthesis” has a disadvantage of the slower kinetics; however, they offer a couple of advantages like better handwork and overgrowth control, and stabilization. These benefits have led to no need for high temperature and pressure as well as toxic chemicals.12–15
The most important use of AgNP is in the medical industry, such as topical ointment to prevent infection in open wounds. Moreover, the antibacterial effect of the AgNP depends on their size, the effect of AgNP decreases when its size is increased.16,17
Materials and Methods
The leaves, trunks, and shells of Taxus brevifolia were collected from the southern mountains of Ziarat village of Gorgan, Iran. Silver nitrate (AgNO3) [Sigma-Aldrich] was applied as an initial source of silver ions as well as an active case study material. Traditional medicine of Taxol (Chemotaxel 30mg/5mL – Paclitaxel injection USP) was prepared as a standard solution for the HPLC experiments.
In order to test the antibacterial activity of AgNP, Staphylococcus aureus (ATCC25923), Escherichia coli (ATCC 1399) and Pseudomonas aeruginosa (ATCC 1430) bacteria were prepared. The human breast cancer cell line MCF-7 was purchased from Pasteur Institute – Cell Bank.
Making Three Extracts from Various Parts of Taxus brevifolia
Several samples of the leaves, trunk, and shells were collected from the Taxus brevifolia tree in order to determine the maximum extractable amount of Taxol in different parts of the tree. The samples were collected and packed in clean drying paper to dry at room temperature. All dried samples were powdered. An equal mass of 20 grams of each dry powdered samples (leaves, trunk, and shells) was boiled separately in 100 mL of double-distilled water. The boiled mixtures were heated in a completely closed container at 40 ºC for 24 hours. The heated materials were filtered through a Whatman No.1 filter paper centrifuged at 5000 rpm for 5 min and a clear solution of the extracts was stored at 4 ° C for further experiments.
High-Performance Liquid Chromatography (HPLC) Experiment
HPLC determined the best extract regarding the highest concentration of Taxol. Different extracts of the Taxus brevifolia tree leaves, stem, and crust were compared to the standard Taxol solution (Chemotaxel 30mg/5mL - Paclitaxel injection USP) using a reverse phase HPLC method (Agilent 1200). 2 μL filtered extract (100.0 μg/mL) applied in a C18 column (Agilent ZORBOX XDB-C18, 250 mm × 4.6 mm) and UV detection was performed at 227 nm as described previously.18 The mobile phase used in this study was a solution containing acetonitrile:water (65:35, v/v) with an adjusted pH of 4.5, according to the protocols.18
Synthesis of Silver Nanoparticles Using T. brevifolia Extract
Based on HPLC experiments, T. brevifolia leaves extract were only used for the synthesis of nanoparticles. Two milliliters of the T. brevifolia leaves extract was mixed with 2 mL of silver nitrate 12.5 mM (0.21 gram/10mL) and was incubated at 37 ° C for one hour. The formation of nanoparticles and the reduction of blue silver ion using the extract of the plant was determined by the color change of the mixture to a yellow-brown (Figure 1).19,20
 |
Figure 1 Silver nanoparticle synthesis using Taxus brevifolia extract. |
Characterization of the Green Synthesized Silver Nanoparticles
It has been reported that the size and shape of nanoparticles mainly depend on different factors like concentration of leaf extract, salt solution, pH, temperature and time.18,19 The obtained AgNPs were washed by centrifuging at 5000g for 45 min, re-suspended in deionized double-distilled water, and washing repeatedly for two times. These were characterized by ultraviolet-visible spectroscopy (UV-VIS), scanning electron microscopy (SEM), X-ray diffraction spectroscopy (XRD) and Fourier-transform infrared spectroscopy (FTIR) as described previously.21–24 In the current work, synthesized AgNPs were measured using UV-Vis spectrophotometry at a range of 350–700 nm (the Agilent 8453 UV-Vis Spectrophotometer, America).
Determination of Antibacterial Activity of Silver Nanoparticles
The synthesized AgNPs were examined for antimicrobial activity by a standard agar diffusion method against different bacterial cultures, S. aureus, E. coli, and P. aeruginosa. An equal number of initial bacterial culture with 0.5 McFarland concentrations was sub-cultured on LB agar. An equal number of initial bacterial culture with 0.5 McFarland concentrations was sub-cultured on LB agar.
All three case study solutions including pure T. brevifolia extract, silver nitrate, and AgNP were diluted individually to obtain seven serial concentrations of titers as follows: (1/1): 50 mM, (1/2): 25 mM, (1/4): 12.5 mM, (1/8): 6.25 mM, (1/16): 3.1 mM, (1/32): 1.5 mM and (1/64): 0.75 mM. Several sets of three selected bacterial culture plates were provided. Equal volumes of 100 μL/well of each dilution were applied to a set of three selected bacterial cultures as described above. Same sets of three bacterial culture plates were considered without any additive material as well as with cephalexin standard antibiogram disks as negative and positive controls, respectively. All plates were evaluated after 24 hours incubation at 37°C. The inhibition zone of the bacteria was accurately measured by the caliper and the mean diameter of the bacterial inhibition zone. The comparison with positive and negative control was considered as an indicator of antibacterial activity evaluation.25 Each assay was repeated three times as described previously.26,27
Minimum Inhibitory Concentration (MIC) Test
The minimum concentration of bacterial growth inhibitory was determined. Same as the previous step, serial dilutions of each case study solutions were prepared. Using a 96-well plate, a set of 7 wells were considered separately for each study materials containing 100 μL of the serial diluents as described above. One hundred microliters of brain–heart-infusion (BHI) broth solution and 100 μL of active bacterial suspension 1.5×106cfu/mL were added to each well. The same procedure was performed for all three bacterial species. Positive and negative controls were considered for each run of the experiments. Positive control wells included the relevant bacteria with BHI solution and negative control included the case study solution (extract, silver nitrate or AgNP) with BHI solution. The plate was incubated for 24 hours at 37°C. The turbidity of the wells, which was due to the growth of the bacteria, was investigated by reading their OD. The first dilution with no turbidity (lack of growth) was recorded as the minimum deterrent concentration.27,28 To reduce the experimental errors, all of the above experiment was repeated four times.
Assessment of Anticancer Activity by MTT Assay
General anti-cancer agents, likewise chemotherapeutic medications or Taxol can suppress cell proliferation; therefore, the viability assay of these agents is expected to show almost the same results on any high proliferating cell line despite the cancerous or normal source of the cells. Consequently, researchers only determine the effects of such remedies on the specific tissue cancer cell lines.2,4
According to the previous step in the present study, 7 dilutions of case study solutions were prepared. The human breast cancer cell line MCF-7 was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. Equal numbers of the cells were passaged in 96-well plates (8×103cells/well) containing 100µL of the medium for 24 hours. One hundred microliters of the different concentrations of the three case study solutions were dispersed in each well and incubated for 24 hours at 37°C with 5% CO2. Fresh medium (100 µL) containing 0.5 mg/mL of MTT was replaced by the previous media in each well. The growth of the cells was quantified by the ability of the live cells to reduce the MTT Pink dye to a pale violet formazan product. After 4 hours, the formazan product of MTT reduction was dissolved in DMSO, and absorbance was measured using a microplate reader. Effect of AgNP as the percentage of control absorbance of reduced dye was measured at 570 nm as well.
Results
Nanoparticle Production HPLC Experiment to Confirmation of Extracts
HPLC experiment was applied for determining Taxol in the extracts. The standard solution of Taxol showed an absorption of 350 mAU at 24–25 minutes after injection (Figure 2A). No peak was observed after injection of the extracts of the crust (Figure 2B) and the stem (Figure 2C) at this time. However, a significant peak of 350 mAU absorption was observed at 24–25 minutes after injection of the leaf extract of yew tree (Figure 2D and E). Therefore, the leaf extract only which was confirmed to have the proper amount of Taxol was applied for the rest of the present study.
In the present study, we applied the green method for producing the AgNP as described in the material and method section. Using ultraviolet-visible spectroscopy (UV-Vis) method, researchers have reported 410 nm wavelength absorption for the AgNP. Our results show the highest SPR absorption band around 410 nm wavelength region (Figure 3A) which confirmed the formation of AgNP.21,29
Surface morphology of the AgNP was studied applying scanning electron microscopy (SEM) with an acceleration voltage of 15kV and 1,04μm resolution (VEGA-TESCAN SEM analyzer) to reveal the size and shape of the produced particles. The SEM analysis displayed the accumulation of the nanoparticles with a size of 15nm. SEM micrographs indicated that the products have been made of inflorescence circular nanoparticles (Figure 3B).
Figure 3C shows the experimental X-ray powder diffraction (XRD) pattern of the prepared AgNP. The diffraction peaks at 38.25, 46.21, 68.32 and 77.34 degrees can be assigned to hexagonal metallic silver corresponding to the (111), (200), (220) and (311) (JCPS file, no. 04–0783) facets of the silver nanoparticle, respectively. The crystallite size was calculated from the width of the XRD peaks, assuming that they were free-form and non-uniform strains, using the Scherrer formula:
D = (0.94 λ)/(β cosθ)
where D is the average crystallite amplitude size perpendicular to the reflecting planes, λ is the X-ray wavelength, β is the full width at half maximum (FWHM), and θ is the diffraction angle.23 Details of the data are given in the supplementary Table S1. The particle size was obtained for three long peaks (depending on the beta and FWHM of each courier) respectively: 7.37, 15.94 and 15.941. The average particle size of the synthesized silver NPs in this work was recorded as 13 nm using the XRD method.
The nature of phytochemicals responsible for the reduction of AgNP and T. brevifolia extract was studied by Fourier-transform infrared (FTIR) spectrometry (Rayleigh WQF-510A spectrometer). The benefit of the FTIR study is the identification of the various functional groups for capping and efficient stabilization of the synthesized metallic nanoparticles. The spectra for characterizing the AgNP were recorded in the range of 4000–400 cm−1 using the KBr pellet method.30
The FTIR spectra bands of the T. brevifolia extract-mediated AgNP of this work are represented in Figure 3D. The observed bands at 3418 and 2920 cm−1 can be assigned to the –OH and aldehyde C–H stretching vibrations, respectively.11 The band located at 2355 cm−1 indicates the N–H stretching/C55O stretching vibrations.31 The band at 1635 cm−1 corresponds to amide I, which arises due to carbonyl stretching vibrations in proteins.32 The characteristic bands observed at 1380 and 1079 cm−1 are attributed to the C55O and C–O stretching vibrations, respectively.31,32 The results demonstrate that the alkaloids may be adsorbed on the surface of metal nanoparticles by a possible interaction via carbonyl groups or π-electrons. The presence of biomass over the surface of AgNP may cause steric or electrostatic barriers, which effectively prevented the accumulation of nanoparticles.
Antibacterial Effect of Nanoparticle Against Three Bacteria
Silver NP solution (Figure 4A) and silver nitrate solution (Figure 4B) had a closed inhibition effect on germ-positive and gram-negative bacteria. However, the Taxus extract solutions did not show antimicrobial effects on three case study bacteria. MIC test results show no antibacterial activity for the pure T. brevifolia extract, where the AgNP showed even slightly more antibacterial effects (MIC: 6.6 mM) comparing the silver nitrate solution (MIC: 6.2 mM) on Staphylococcus aureus. Average MICs were recorded as 1.5 mM for E. coli, and 1.5 mM for Pseudomonas applying silver nitrate and AgNP (Table S2).
 |
Figure 4 The well test. (A) Effects of the silver nanoparticle on three types of bacteria. (B) Effects of silver nitrate on three types of bacteria. |
Assessment of Anticancer Activity by MTT Assay and DAPI Staining
Silver nitrate solution was observed to have more cytotoxic activity compared to the pure T. brevifolia extracts and synthesized AgNP. T. brevifolia extract is a known anti-cancer remedy by a mechanism of cell division prevention likewise chemotherapeutic reagents. Obviously, the cells with higher proliferation are more affected by such a remedy. The anticancer activity of new synthesized AgNP tends to be more effective in more concentrated solutions. In the 25 mM of silver nanoparticle, about 78% of cancer cells are dead. Extract in the half dilution state showed the most anti-cancer activity where 72% of cancer cells die. All dilutions of silver nitrate solution exhibited closed anticancer activities with an 82–86% of cancer cells’ mortality rate (Figure 5). Breast cell proliferation inhibitory activity of T. brevifolia extract, silver nitrate, and silver NPs demonstrated in the supplementary Table S3. DAPI staining is a method to detect cell death in which the dye can infiltrate the nucleus of the dead cells only. DAPI staining reviled a high rate of apoptosis of the breast cancer cells following the treatment with the nanoparticles (Figure 6).
 |
Figure 5 Comparing anticancer effect of new AgNP, Taxol and silver nitrate solution using MTT assay on the breast cancer cells. |
 |
Figure 6 DAPI staining approved the apoptotic effects of the silver nanoparticles on the breast cancer cells regarding the infiltration of the nucleus by the dye (arrows). |
TEM Imaging and DLS Confirmation
The transmission electron microscope (TEM) image and dynamic light scattering (DLS) techniques were applied to determining the shape and size of the nanoparticles. TEM measurements were performed on a Zeiss model EM900 instrument operated at 80 kV accelerating voltage. The green method synthesized AgNP were prepared for TEM measurements by placing a drop over carbon-coated copper grids and allowing the solvent to be evaporated. In the present study, the morphology of the AgNP, which was observed through the TEM micrograph, was hexagonal (Figure 7A). This hexagonal shape for the green method synthesized AgNP has been reported previously using the sunlight as reducing agent as well.33 The TEM micrograph revealed a range of 5 to 25nm for the produced particles. This range was confirmed using the DLS technique too (Figure 7B).
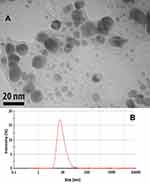 |
Figure 7 Determination of the shape and size of the silver nanoparticles synthesized by Taxus brevifolia extract. (A) Transmission electron microscope (TEM). (B) Dynamic light scattering (DLS). |
Discussion
The Taxus tree has been proven to contain an anti-cancerous substance called Taxol. In the present study, three different samples from leaves, trunk, and shells of T. brevifolia tree were used to prepare the extracts. HPLC experiments revealed more Taxol content in the T. brevifolia leaves extract.
Consequently, AgNPs were synthesized by T. brevifolia leaves extract using the green method synthesis. Antimicrobial and anticancer effects of T. brevifolia leaves extract, silver nitrate and AgNP were compared. In conclusion, the synthesis of AgNP using T. brevifolia leaves extract showed good results for biomedical applications. An absorption peak at 410 nm in the UV-vis spectrum proved the formation of silver NPs. Moldovan also reported a 407 nm absorption peak in UV–vis spectrometry for the silver nanoparticle which is synthesized with European black elderberry extract by the same method.33 The obtained results from UV-vis spectrophotometry, as well as SEM, and XRD of AgNP confirmed the efficiency of T. brevifolia leaves extract in the synthesis of hexagonal AgNP. Same methods have been applied to confirm the synthesis of AgNPs.34 Taruna et al reported spherical silver NPs and Sinha & Paul cubic silver NPs synthesized with green methods.18,35 The FTIR spectrum of the silver NPs and the extract has shown reduction functional groups for silver ion reduction. The functional groups for reducing metal ions in any tree or plant are different. The detected functional groups are alkaloids such as Taxanes A and Taxanes B in the extract of T. brevifolia leaves, as well as hydroxyflavones and catechins in the extract of papaya fruit,36 and molecules with hydroxylamine factor in the seed extract of Punica granatum.37
Metal-based nanoparticles have many free electrons that move by conduction and balance bands which are caused by surface plasmon resonance (SPR) after the UV light collision to them. The spectrum of this resonance records the vibrations of the free electrons of the nanoparticle.38,39 Khodadadi et al also have described the same method to confirm the formation of silver NPs with Achillea millefolium L. extract using the green method nanoparticle synthesis.40 In the present study, the results from high-throughput techniques such as UV-visible spectroscopy, FTIR, SEM, and XRD measurements indicated the successful formation of Silver NPs.
The antibacterial activity experiment revealed the highest inhibition for S. aureus bacteria, which was recorded as 20mm at 50mM, 18mm at 25mM, 16mm at 12.5mM, 14mm at 6.25mM, 13mm at 3.1mM, and 12mm at 1.5 mM concentration of AgNP and silver nitrate solution. No inhibitory effect observed around the wells at 0.75 mM concentration of AgNP and silver nitrate solution. Furthermore, there was no bacterial inhibition by AgNP and silver nitrate solution in E.coli and P. aeruginosa plates. The T. brevifolia extract did not show any antibacterial effects.
Previous works reviewed several mechanisms of AgNP antibacterial effects, in which the release of silver ions from the nanoparticle surface is an essential step in all mechanisms.41,42 The MIC test in this study revealed a slightly more antibacterial effect of the silver nanoparticle. This MIC similarity can indicate that there are no ion-releasing suppression effects on AgNP produced by adding T. brevifolia extract.
Anticancer activity of T. brevifolia extract, silver nitrate, and AgNP was investigated. T. brevifolia extract is a known anti-cancer remedy. The anticancer activity of the new synthesized AgNP tends to be more effective in more concentrated solutions. MTT assay showed that bio-synthesized Silver NPs had a cytotoxic effect on human breast cancer cell line MCF-7 like silver nitrate solution. According to the recorded results, one of the AgNP solutions which had the most anticancer activity was selected (25 mM). DAPI staining confirmed the apoptotic effects of AgNP on the breast cancer cells in terms of the infiltration of the nucleus by the dye. The TEM images of this solution confirmed the presence of nanoparticles with a size range of 5–25 nm.
Although AgNPs are well known as an antimicrobial agent,43,44 however, several scientists have described applications like an anticancer,7,45 biosensor,46 and water microbial filters47 (Supplementary Table S4). The antimicrobial effect of silver NPs may be due to either (i) the formation of holes in the cell wall, which ultimately leads to leakage of the cellular cytoplasmic content or (ii) the silver ions change the ribosome and inhibits the expression of the enzymes and thiol including proteins essential for the generation of ATP and DNA thus resulting in cell death.48,49 Furthermore, researchers reported the interaction of silver NPs with HIV-1 by binding to gp120 glycoprotein knobs. This type of interaction of silver NPs specifically inhibits the binding of the virus to host cells.48 The plasmonic properties of silver NPs belong to the shape, size and dielectric medium that surrounds those.50 Therefore, this dependency can conduct the applicability of silver NPs in biosensing.46 They suggest a size range of 12–50 nm with a Neutral Surface charge. The average particle size of the synthesized silver NPs in the present study was recorded as 13 nm using an XRD method with a range of 5–25 nm according to the TEM imaging.
Prohibiting the growth of harmful microorganisms by improving or covering the surfaces with antimicrobial agents has received serious consideration for application in biomedical devices and health, as well as in the food and hygiene industries.47 This application should possess adequate antibacterial benefits along with lower toxicity for humans. Silver nitrate solution shows a severe dose-independent cell toxicity in our results (Figure 5). Silver nanoparticle seems to have lower toxicity and residues in water, food, as well as less systemic absorption from topical remedies comparing silver ion-containing solutions such as silver nitrate.51–53
Conclusion
As an overall view, the present study proposed more anticancer, as well as antibacterial effects for AgNP made by Taxus brevifolia extract, comparing Taxol solution. Moreover, it revealed a dose-dependent biocompatibility along with slightly more antibacterial effects for AgNP compared with silver nitrate solution. The production of herbal-mediated silver nanoparticles may be an efficient substitution for the silver nitrate–based medicines with fewer side effects.
Acknowledgments
The authors gratefully appreciate Mrs Javid and Mr Bazouri, research scientists at the Department of Microbiology, and Dr M. Sheikharabi at the department of Medical Nanotechnology, GoUMS for their assistance in this work. This research was conducted at the laboratory of the Department of Microbiology, GoUMS.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–2702. doi:10.1039/C8RA08982E
2. Kim S, Park SG, Song YJ, et al. Analysis of anticancer activity and chemical sensitization effects of Dendropanax morbifera and Commersonia bartramia extracts. Anticancer Res. 2018;38(7):3853–3861. doi:10.21873/anticanres.12669
3. Kingston DGI. The shape of things to come: structural and synthetic studies of taxol and related compounds. Phytochemistry. 2007;68(14):1844–1854. doi:10.1016/j.phytochem.2006.11.009
4. Kingston DGI, Samranayake G, Ivey CA. The chemistry of taxol, a clinically useful anticancer agent. J Nat Prod. 1990;53(1):1–12. doi:10.1021/np50067a001
5. JNoel D, Greene AE, Daniel G, Francoise G-V, Lydie M, Pierre P. Highly efficient, practical approach to natural taxol. J Am Chem Soc. 1988;110(17):5917–5919. doi:10.1021/ja00225a063
6. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93(9):2325–2327. doi:10.1021/ja00738a045
7. Kajani AA, Bordbar A-K, Zarkesh Esfahani SH, Khosropour AR, Razmjou A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014;4(106):61394–61403. doi:10.1039/C4RA08758E
8. Shankar Naik B. Developments in taxol production through endophytic fungal biotechnology: a review. Orient Pharm Exp Med. 2019;19(1):1–13. doi:10.1007/s13596-018-0352-8
9. Singh H, Du J, Yi T-H. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells Nanomedicine Biotechnol. 2017;45(7):1310–1316. doi:10.1080/21691401.2016.1228663
10. Carmona ER, Benito N, Plaza T, Recio-Sánchez G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem Lett Rev. 2017;10(4):250–256. doi:10.1080/17518253.2017.1360400
11. Shabestarian H, Homayouni-Tabrizi M, Soltani M, et al. Green synthesis of gold nanoparticles using sumac aqueous extract and their antioxidant activity. Mater Res. 2017;20(1):264–270. doi:10.1590/1980-5373-mr-2015-0694
12. Hosseini SS, Ghaemi E, Noroozi A, Niknejad F. Zinc oxide nanoparticles inhibition of initial adhesion and ALS1 and ALS3 gene expression in candida albicans strains from urinary tract infections. Mycopathologia. 2019;184(2):261–271. doi:10.1007/s11046-019-00327-w
13. Hosseini SS, Ghaemi E, Koohsar F. Influence of ZnO nanoparticles on Candida albicans isolates biofilm formed on the urinary catheter. Iran J Microbiol. 2018;10(6):424–432.
14. Nakkala JR, Mata R, Sadras SR. Green synthesized nano silver: synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J Colloid Interface Sci. 2017;499:33–45. doi:10.1016/j.jcis.2017.03.090
15. Saravanakumar A, Peng MM, Ganesh M, Jayaprakash J, Mohankumar M, Jang HT. Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif Cells Nanomedicine Biotechnol. 2017;45(6):1165–1171. doi:10.1080/21691401.2016.1203795
16. Khatami M, Sharifi I, Nobre MAL, Zafarnia N, Aflatoonian MR. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem Lett Rev. 2018;11(2):125–134. doi:10.1080/17518253.2018.1444797
17. Nabikhan A, Kandasamy K, Raj A, Alikunhi NM. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B Biointerfaces. 2010;79(2):488–493. doi:10.1016/j.colsurfb.2010.05.018
18. Sinha SN, Paul D. Photosynthesis of silver nanoparticles using Andrographis paniculata leaf extract and evaluation of their antibacterial activities. Spectrosc Lett. 2015;48(8):600–604. doi:10.1080/00387010.2014.938756
19. Rivera-Rangel RD, González-Muñoz MP, Avila-Rodriguez M, Razo-Lazcano TA, Solans C. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf Physicochem Eng Asp. 2018;536:60–67. doi:10.1016/j.colsurfa.2017.07.051
20. Tse ECM, Gewirth AA. Effect of temperature and pressure on the kinetics of the oxygen reduction reaction. J Phys Chem A. 2015;119(8):1246–1255. doi:10.1021/acs.jpca.5b00572
21. Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C. 2016;58:36–43. doi:10.1016/j.msec.2015.08.018
22. Saha M, Bandyopadhyay PK. Green biosynthesis of silver nanoparticle using garlic, allium sativum with reference to its antimicrobial activity against the pathogenic strain of bacillus sp. and pseudomonas sp. infecting Goldfish, Carassius auratus. Proc Zool Soc. 2017. doi:10.1007/s12595-017-0258-3
23. Suresh D, Nethravathi PC, Udayabhanu RH, Nagabhushana H, Sharma SC. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater Sci Semicond Process. 2015;31:446–454. doi:10.1016/j.mssp.2014.12.023
24. Vinayagam R, Varadavenkatesan T, Selvaraj R. Green synthesis, structural characterization, and catalytic activity of silver nanoparticles stabilized with Bridelia retusa leaf extract. Green Process Synth. 2018;7(1):30–37. doi:10.1515/gps-2016-0236
25. Shivakumar M, Nagashree KL, Yallappa S, Manjappa S, Manjunath KS, Dharmaprakash MS. Biosynthesis of silver nanoparticles using pre-hydrolysis liquor of Eucalyptus wood and its effective antimicrobial activity. Enzyme Microb Technol. 2017;97:55–62. doi:10.1016/j.enzmictec.2016.11.006
26. Almeida AAP, Farah A, Silva DAM, Nunan EA, Glória MBA. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem. 2006;54(23):8738–8743. doi:10.1021/jf0617317
27. Sri Sindhura K, Prasad TNVKV, Panner Selvam P, Hussain OM. Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl Nanosci. 2014;4(7):819–827. doi:10.1007/s13204-013-0263-4
28. Qu J, Yuan X, Wang X, Shao P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ Pollut. 2011;159(7):1783–1788. doi:10.1016/j.envpol.2011.04.016
29. Dos Santos Junior VE, Targino AGR, Flores MAP, et al. Antimicrobial activity of silver nanoparticle colloids of different sizes and shapes against Streptococcus mutans. Res Chem Intermed. 2017;43(10):5889–5899. doi:10.1007/s11164-017-2969-5
30. Shankar T, Karthiga P, Swarnalatha K, Rajkumar K. Green synthesis of silver nanoparticles using Capsicum frutescence and its intensified activity against E. coli. Resour-Effic Technol. 2017;3(3):303–308. doi:10.1016/j.reffit.2017.01.004
31. Ramachandran K, Kalpana D, Sathishkumar Y, Lee YS, Ravichandran K, Kumar GG. A facile green synthesis of silver nanoparticles using Piper betle biomass and its catalytic activity toward sensitive and selective nitrite detection. J Ind Eng Chem. 2016;35:29–35. doi:10.1016/j.jiec.2015.10.033
32. Hu G, Cai Y, Tu Z, et al. Reducing the cytotoxicity while improving the anti-cancer activity of silver nanoparticles through α-tocopherol succinate modification. RSC Adv. 2015;5(100):82050–82055. doi:10.1039/C5RA12911G
33. Moldovan B, David L, Achim M, Clichici S, Filip GA. A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L. fruits extract and their antioxidant activity. J Mol Liq. 2016;221:271–278. doi:10.1016/j.molliq.2016.06.003
34. Shaban SM, Aiad I, El-Sukkary MM, Soliman EA, El-Awady MY. One step green synthesis of hexagonal silver nanoparticles and their biological activity. J Ind Eng Chem. 2014;20(6):4473–4481. doi:10.1016/j.jiec.2014.02.019
35. Taruna KJ, Bhatti J, Kumar P. Green synthesis and physico-chemical study of silver nanoparticles extracted from a natural source Luffa acutangula. J Mol Liq. 2016;224:991–998. doi:10.1016/j.molliq.2016.10.065
36. Mude N, Ingle A, Gade A, Rai M. Synthesis of silver nanoparticles using callus extract of carica papaya — a first report. J Plant Biochem Biotechnol. 2009;18(1):83–86. doi:10.1007/BF03263300
37. Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50(17):4791–4795. doi:10.1021/jf0255735
38. Evanoff DD, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem. 2005;6(7):1221–1231. doi:10.1002/cphc.200500113
39. Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B. 2003;107(3):668–677. doi:10.1021/jp026731y
40. Khodadadi B, Bordbar M, Nasrollahzadeh M. Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: catalytic activity for reduction of organic dyes. J Colloid Interface Sci. 2017;490:1–10. doi:10.1016/j.jcis.2016.11.032
41. Rafique M, Sadaf I, Rafique MS, Tahir MB. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomedicine Biotechnol. 2017;45(7):1272–1291. doi:10.1080/21691401.2016.1241792
42. Rónavári A, Kovács D, Igaz N, et al. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int J Nanomedicine. 2017;12:871–883. doi:10.2147/IJN.S122842
43. Ahmad N, Sharma S, Singh VN, Shamsi SF, Fatma A, Mehta BR. Biosynthesis of silver nanoparticles from desmodium triflorum: a novel approach towards weed utilization. Biotechnol Res Int. 2011;2011:1–8. doi:10.4061/2011/454090
44. Banasiuk R, Krychowiak M, Swigon D, et al. Carnivorous plants used for green synthesis of silver nanoparticles with broad-spectrum antimicrobial activity. Arab J Chem. 2020;13(1):1415–1428. doi:10.1016/j.arabjc.2017.11.013
45. Premkumar T, Lee Y, Geckeler KE. Macrocycles as a tool: a facile and one‐pot synthesis of silver nanoparticles using cucurbituril designed for cancer therapeutics. Chem- Eur J. 2010;16(38):11563–11566. doi:10.1002/chem.201001325
46. Loiseau A, Asila V, Boitel-Aullen G, Lam M, Salmain M, Boujday S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors. 2019;9(2):78. doi:10.3390/bios9020078
47. Ali Z, Ahmad R. Nanotechnology for water treatment. In: Dasgupta N, Ranjan S, Lichtfouse E, editors. Environmental Nanotechnology Volume 3. Vol. 27. Cham: Springer International Publishing; 2020: 143–163. doi:10.1007/978-3-030-26672-1_5.
48. Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720. doi:10.1128/AEM.02218-06
49. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi:10.1016/j.jcis.2004.02.012
50. Zhang S, Bao K, Halas NJ, Xu H, Nordlander P. Substrate-induced fano resonances of a plasmonic nanocube: a route to increased-sensitivity localized surface plasmon resonance sensors revealed. Nano Lett. 2011;11(4):1657–1663. doi:10.1021/nl200135r
51. Ahmad A, Wei Y, Syed F, et al. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb Pathog. 2017;102:133–142. doi:10.1016/j.micpath.2016.11.030
52. Kumar I, Mondal M, Sakthivel N. Green synthesis of phytogenic nanoparticles. In: Shukla AK, Iravani S, editors. Green Synthesis. Characterization and Applications of Nanoparticles. Elsevier; 2019:37–73. doi:10.1016/B978-0-08-102579-6.00003-4
53. Nasirian V, Chabok A, Barati A, Rafienia M, Arabi MS, Shamsipur M. Ultrasensitive aflatoxin B1 assay based on FRET from aptamer labelled fluorescent polymer dots to silver nanoparticles labeled with complementary DNA. Microchimica Acta. 2017;184(12):4655–4662. doi:10.1007/s00604-017-2508-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


