Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
A Phase II single-arm trial of palonosetron for the prevention of acute and delayed chemotherapy-induced nausea and vomiting in malignant glioma patients receiving multidose irinotecan in combination with bevacizumab
Authors Affronti ML, Woodring S, Peters KB, Herndon II JE, McSherry F, Healy PN, Desjardins A, Vredenburgh JJ, Friedman HS
Received 16 September 2016
Accepted for publication 17 November 2016
Published 23 December 2016 Volume 2017:13 Pages 33—40
DOI https://doi.org/10.2147/TCRM.S122480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Mary Lou Affronti,1–3 Sarah Woodring,1,2 Katherine B Peters,1,4 James E Herndon II,5 Frances McSherry,5 Patrick N Healy,5 Annick Desjardins,1,4 James J Vredenburgh,6 Henry S Friedman1,2
1The Preston Robert Tisch Brain Tumor Center at Duke, South Hospital, Duke University Medical Center, 2Department of Neurosurgery, Duke University Health System, 3Duke University School of Nursing, 4Department of Neurology, 5Department of Biostatistics and Bioinformatics, Duke University Health System, Durham, NC, 6Saint Francis Cancer Center, Hartford, CT, USA
Purpose: Given that the prognosis of recurrent malignant glioma (MG) remains poor, improving quality of life (QoL) through symptom management is important. Meta-analyses establishing antiemetic guidelines have demonstrated the superiority of palonosetron (PAL) over older 5-hydroxytryptamine 3-receptor antagonists in chemotherapy-induced nausea and vomiting (CINV) prevention, but excluded patients with gliomas. Irinotecan plus bevacizumab is a treatment frequently used in MG, but is associated with low (55%) CINV complete response (CR; no emesis or use of rescue antiemetic) with commonly prescribed ondansetron. A single-arm Phase II trial was conducted in MG patients to determine the efficacy of intravenous PAL (0.25 mg) and dexamethasone (DEX; 10 mg) received in conjunction with biweekly irinotecan–bevacizumab treatment. The primary end point was the proportion of subjects achieving acute CINV CR (no emesis or antiemetic ≤24 hours postchemotherapy). Secondary end points included delayed CINV CR (days 2–5), overall CINV CR (days 1–5), and QoL, fatigue, and toxicity.
Materials and methods: A two-stage design of 160 patients was planned to differentiate between CINV CR of 55% and 65% after each dose of PAL–DEX. Validated surveys assessed fatigue and QoL.
Results: A total of 63 patients were enrolled, after which enrollment was terminated due to slow accrual; 52 patients were evaluable for the primary outcome of acute CINV CR. Following PAL–DEX dose administrations 1–3, acute CINV CR rates were 62%, 68%, and 70%; delayed CINV CR rates were 62%, 66%, and 70%, and overall CINV CR rates were 47%, 57%, and 62%, respectively. Compared to baseline, there was a clinically meaningful increase in fatigue during acute and overall phases, but not in the delayed phase. There were no grade ≥3 PAL–DEX treatment-related toxicities.
Conclusion: Data suggest that PAL–DEX is effective in preventing CINV in MG patients, which ultimately maintains the QoL of patients with glioma.
Keywords: chemotherapy, nausea, chemotherapy-induced nausea and vomiting, antiemetic guidelines, evidence-based practice, glioma
Introduction
Chemotherapy-induced nausea and vomiting (CINV) continues to be one of the most debilitating side effects of cancer therapy, despite dramatic advances in antiemetics.1,2 Although effective evidence-based guidelines are available, CINV prevention in cancer patients receiving antineoplastic therapy remains suboptimal, because providers often do not adhere to practice guidelines.3 Available antiemetics can prevent up to 90% of CINV, but 60%–80% of patients continue to experience NV, which negatively impacts quality of life (QoL).4–8
CINV can be classified as acute CINV (NV occurring within 24 hours postchemotherapy), and delayed CINV (NV occurring ≥24–120 hours postchemotherapy). Short-acting 5-hydroxytryptamine 3 (5-HT3)-receptor antagonists (RAs), the most frequently used antiemetics, are effective in preventing acute CINV, but less effective in ameliorating delayed CINV.4,9–11 Providers underestimate the incidence of delayed CINV, resulting in inadequate management of overall CINV.4,5,12
Published meta-analyses demonstrate that the second-generation long-acting 5-HT3-RA single-dose palonosetron (PAL; Eisai Inc, Woodcliff Lake, NJ, USA) or equivalent multidose ramosetron with higher serotonin selectivity is superior to first-generation 5-HT3-RAs in preventing both acute and delayed CINV.13–16 Unique advantages of PAL over older 5-HT3-RAs include longer half-life, higher 5-HT3-receptor binding, and ability to inhibit cross talk between 5-HT3 and neurokinin 1-signaling pathways.17–20 Patients receiving PAL with moderately emetic chemotherapy (MEC) have similar minor toxicities and fewer acute and delayed NV episodes than patients receiving short-acting 5-HT3-RAs.14
The emetogenicity of chemotherapy regimens varies. MEC causes CINV in >30% of patients; highly emetic agents can cause CINV in >90% of patients. Current National Comprehensive Cancer Network and American Society of Clinical Oncology antiemetic guidelines for patients receiving MEC regimens recommend combining PAL (“preferred” 5-HT3-RA) with dexamethasone (DEX) to prevent both acute and delayed CINV.21,22 These guidelines are supported by meta-analyses of high-level evidence. However, patients with malignant glioma (MG) were excluded from these larger studies, due to brain pathology, seizure potential, and glioma–drug interactions. For MG patients with CINV, PAL plus DEX antiemetic guidelines are supported only by small studies.23 Neurokinin 1-RAs are often avoided in MG patients, because they interact with certain medications (eg, DEX, enzyme-inducing antiepileptic drugs [EIAEDs]) used in this population.23,24
Given the poor prognosis of recurrent MG (median survival is 3–9 months), improving health-related QoL through symptom management is an important goal. Once-a-week PAL dosing is expected to increase antiemetic efficacy in MG patients, whose antiemetic compliance is often compromised by memory deficits. Older 5HT3-RA antiemetics are less expensive, but this benefit is offset by PAL’s ability to reduce the risk of extreme CINV events (eg, rehospitalizations due to dehydration), thus substantially reducing overall costs and staff time required to manage delayed CINV.25 The majority of patients receiving PAL have higher functionality with no significant impact on QoL compared to patients treated with older 5HT3-RAs.26
Study objective
Irinotecan in combination with bevacizumab (BEV) is an effective MEC regimen for MG, but is associated with a low CINV complete response (CR) of 55% with ondansetron. Therefore, a single-arm Phase II trial was conducted to determine the efficacy of intravenous PAL (0.25 mg) plus DEX (10 mg) in MG patients receiving biweekly irinotecan–BEV treatment. The primary end point was the proportion of patients achieving acute CINV CR (no emesis or antiemetic ≤24 hours postchemotherapy). Secondary end points included delayed CINV CR (days 2–5), overall CINV CR (days 1–5), QoL, fatigue, and toxicity.
Materials and methods
Study design
In several Phase II glioma studies of irinotecan–BEV conducted in the authors’ institution (Duke-Institutional Review Board Pro00002273), patients were treated with ondansetron (first-generation 5HT3-RA) for CINV prevention. Approximately 55%–60% of patients experienced no emetic episode and needed no rescue medications.24 A pilot survey suggested that 60% of patients experience no emetic episode on ondansetron; however, most of these patients were on a significant amount of oral DEX (an antiemetic), which probably added to the overall CR rate.24 Therefore, 55% was used as the acute and overall CR rate for historical control patients on ondansetron plus DEX.
To assess efficacy of PAL treatment in this study, a two-phase design27 was planned. If the true acute CINV CR rate for patients receiving PAL plus DEX is ≤55%, there would be little interest in adopting this regimen as standard treatment in patients receiving chemotherapy for MG. However, if the true CINV CR rate is ≥65%, use of this regimen in MG patients merits further exploration. Therefore, in this study, there was interest in differentiating between acute CINV CR rates of 55% and 65%. The variable to be tested was proportion <0.55 versus proportion >0.65, where “proportion” represents the proportion of patients who do not experience CINV during the first 24 hours of chemotherapy treatment and do not need rescue medication.
It was anticipated that 80 patients would be accrued during the first stage of the study, after which one of three actions would be taken. If <44 patients had an acute CINV CR, accrual would be terminated and the treatment rejected as ineffective in preventing CINV in MG patients. If ≥53 patients had an acute CINV CR, accrual would be terminated and the effectiveness of the regimen in treating MG patients would be accepted. Otherwise, an additional 80 patients would be accrued to the study.
Eligibility criteria
Patients aged ≥18 years with histologically confirmed malignant (or progressive low-grade) glioma who were scheduled to receive multidose irinotecan–BEV every 2 weeks for one 6-week cycle were eligible for the study. Study inclusion criteria were: 1) interval of >6 weeks from surgery and >4 weeks from radiotherapy, 2) Karnofsky Performance Scale score ≥60%, 3) stable dose of steroids 1 week prior to entry, 4) adequate blood count and renal and hepatic function, and electrolytes within normal limits, 5) no evidence of central nervous system hemorrhage on baseline magnetic resonance imaging or computed tomography, and 6) agreement by sexually active patients to use contraceptive measures for the duration of treatment. Exclusion criteria were: 1) received any intravenous drug with potential antiemetic effect within 24 hours before the start of the study, 2) any vomiting, retching, or National Cancer Institute common toxicity criteria grade 2–4 nausea during 24 hours preceding chemotherapy, 3) received PAL <14 days prior to study enrollment, 4) comedication (other than corticosteroids for cerebral swelling) interfering with study results, and 5) homozygosity of the *28 polymorphism of the UGT1A1 gene (refers specifically to TA7, not TA6). The protocol for this study was approved by the Duke University Health System Institutional Review Board, and each patient signed informed consent.
Treatment plan
Patients received intravenous PAL 0.25 mg and DEX 10 mg (hereafter PAL–DEX) 30 minutes prior to the MEC regimen of irinotecan in combination with BEV 5–10 mg/kg. Patients received standard diarrhea prophylaxis (loperamide/atropine). This MEC regimen was delivered every other week, for a total of three doses in a 6-week cycle (Table 1). The irinotecan dose was 340 mg/m2 for patients taking EIAEDs, such as phenytoin, carbamazepine, and phenobarbital. Patients taking non-EIAEDs and those taking no AEDs received irinotecan at 125 mg/m2. Irinotecan dose was reduced by 25% if a patient experienced grade 3 or 4 gastrointestinal or hematological toxicity; patients were taken off the study if they experienced grade 3 or 4 toxicity at the reduced dose.
CINV assessment
The objective of this study was to assess the efficacy of PAL–DEX in preventing CINV in glioma patients receiving an MEC regimen combining irinotecan and BEV (hereafter irinotecan–BEV). CINV was assessed by reviewing patient diaries, which were used to record nausea, vomiting, or rescue medication taken during the 5 days after each of the three PAL–DEX doses.
The primary end point for this assessment was the proportion of patients with an acute CINV CR, defined as no emetic episode and no antiemetic rescue medication during the first 24 hours after chemotherapy administration, determined from reports in patient diaries. Delayed CINV CR and overall CINV CR rates – defined respectively as the proportions of patients achieving CR during the delayed (>24–120 hours) and the overall (0–120 hours) periods following chemotherapy administration – were calculated in secondary analyses. Acute, delayed, and overall CR rates were also calculated separately for chemotherapy-induced vomiting (CIV CR; defined as having no vomiting episodes) and for CIN (CIN CR, defined as having no nausea episodes).
Toxicity and QoL assessment
Toxicity grading was conducted using the National Cancer Institute’s Common Toxicity Criteria for Adverse Events, version 3.0,28 and the frequency of patients experiencing adverse events was summarized using the maximum grade of each type of toxicity experienced. A survey including three validated, reliable measures of CINV-related QoL outcomes29 was administered to each patient at baseline, day 1 (acute CINV), and days 2–5 (delayed CINV) of each dose (Table 1). The survey, which took 10–15 minutes to complete, included the Modified Functional Living Index–emesis (M-FLIE),30,31 Functional Assessment of Chronic Illness Therapy-fatigue (FACIT-F),32 and Martin et al’s NV-5 instruments.33 The M-FLIE measures functional impact of nausea (nine items) and vomiting (nine items) on daily life; total scores range from 18 to 126, and higher scores indicate better QoL.30,31,34 The 13-item FACIT-F assesses the impact of fatigue on QoL; total scores range from 0 to 52, higher values indicate better QoL, and a ±3-point change in score from baseline is considered clinically meaningful.32 The NV-5 includes separate modules for nausea and vomiting/retching. Patients rate the impact of each symptom in five QoL domains, and summed ratings within each module are converted to a standardized 0–100 scale, with 0 indicating no symptom impact on any QoL domain and high scores indicating severe symptom impact on multiple domains.33
To assess CINV effects on QoL (M-FLIE) and fatigue (FACIT-F), change scores were calculated for baseline vs day 1 (acute), baseline vs average score for days 2–5 (delayed), and baseline vs average score for days 1–5 (overall). Effects of nausea and vomiting on QoL were assessed separately by determining the percentage of patients with a standardized score of 0 on each NV-5 module for day 1 (acute) and days 2–5 (delayed) after each PAL–DEX dose.
Results
Patient characteristics
After enrollment of 63 patients, the study was ended early due to low accrual rate. Patient characteristics are summarized in Table 2. Mean age was 53.2 (standard deviation: 13.1, range: 28–75) years, 67% were males, and 92% were Whites. Seventy percent were diagnosed with glioblastoma (World Health Organization grade IV), 52% had Karnofsky Performance Scale score ≥90%, 27% were taking EIAEDs, and 36.5% used oral steroids. Of note, there were no statistical differences in CINV CR rates by baseline steroid use.
CINV risk factors
The majority of patients in this study had at least one risk factor for CINV: 51% reported prior chemotherapy, and 62% reported never using alcohol. With respect to other risk factors, 33% were females, 41% were aged <50 years, 49% had a prior history of CINV, 13% had a prior history of motion sickness, and 14% had prior history of morning sickness.
CINV complete response
A total of 52 patients were evaluable for the primary outcome of acute CINV CR (percentage of patients with no vomiting or antiemetic rescue by dose); their CINV CR rates are summarized in Tables 3 and 4. For MEC and PAL–DEX dose administrations 1–3 (hereafter referred to as doses 1–3), acute CINV CR rates were 62%, 68%, and 70%, respectively, delayed CINV CR rates were 62%, 66%, and 70%, respectively, and overall CINV CR rates were 47%, 57%, and 62%, respectively (Table 3).
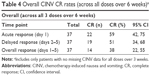
Table 3 also presents acute, delayed, and overall CR rates for CIV (percentage of patients with no vomiting event) and CIN (percentage of patients reporting no nausea) for each dose. Acute CR rates for doses 1–3 were higher for CIV (89%, 91%, and 89%, respectively) than CIN (60%, 66%, and 59%, respectively). Delayed and overall CR rates were also higher for CIV than CIN. Overall CINV CR rates pooled across all three doses were 59% for acute, 51% for delayed, and 38% for overall response (Table 4).
Toxicity
Overall, patients tolerated PAL well: 12% experienced mild-to-moderate PAL-related toxicities (mild headache 2%, diarrhea 5%, constipation 5%), and there were no grade ≥3 PAL-related toxicities. The vast majority of reported adverse events were attributable to the chemotherapy regimen or the underlying disease, and not to PAL: 41% (27 of 63) of patients experienced a grade ≥3 non-PAL-related adverse event. Four patients were hospitalized (three with infections, one with grade 4 fatigue due to grade 3 diarrhea), and one hospitalized patient died due to Klebsiella pneumonia.
Health-related quality of life and fatigue
Mean scores on the M-FLIE and FACIT-F during the 5 days following dose 1 are shown in Tables 5 and 6. M-FLIE scores were eleven points below baseline on day 1 after dosing (indicating some worsening in daily functioning due to CINV during the acute phase), and showed improvement in the delayed phase, with scores returning to near-baseline levels by day 5 (Table 5). M-FLIE scores after doses 2 and 3 (not presented) followed similar patterns. Mean FACIT-F scores dropped 5.6 points on day 1 after dosing (indicating a clinically meaningful increase in fatigue during the acute phase, as per Cella et al),32 but showed partial recovery during days 2–5 (Table 6). FACIT-Fatigue results were similar after doses 2 and 3 (not presented). NV-5 data for dose 1 (Table 7) indicated that 37% of patients reported that nausea reduced QoL on day 1 (38% during days 2–5), but only 12% reported that retching/vomiting reduced QoL on day 1 (13% during days 2–5); results were similar for doses 2 and 3.
Discussion
Individuals with malignant primary brain tumors are often excluded from antiemetic clinical trials, due to brain pathology, steroid use, and potential for drug interactions with standard glioma medications. Therefore, the generalizability of robust trial results to this patient population remains limited. To date, one small (33-patient) Phase II trial has documented that the guideline antiemetic PAL is effective in nonrecurrent patients receiving adjuvant temozolomide therapy.23 Acute, delayed, and overall CINV CR rates were 88%, 91%, and 88%, respectively, in newly diagnosed glioma patients receiving standard multidose temozolomide (150–200 mg/m2/day) for 5 days; grade 1–2 headache (21%) was the most frequent adverse event.23
The paucity of evidence-based antiemetic literature in the recurrent glioma patient population is complicated by low provider adherence to prescribing guideline antiemetics to prevent CINV. Implementing a combination intervention (educational in-service, standardized guideline antiemetic order sets) and audit-feedback system significantly increased prescription of PAL–DEX guideline antiemetics (from 58% baseline to sustained 90%), resulting in significant improvement of both acute (75%) and delayed (84%) CINV CR rates while maintaining QoL.35 One goal of this Phase II trial was thus to add to the guideline CINV literature by determining the efficacy of treating MG patients receiving MEC with intravenous PAL (0.25 mg)–DEX (10 mg).
The majority of glioma patients enrolled had two major CINV risk factors: prior treatment with chemotherapy (51%), and no history of alcohol use (62%). CINV CR rates were higher than the historical 55% control rate for multidose cycles: acute CR rates were 62%, 68%, and 70%, and delayed CR rates of 62%, 66%, and 70% for doses 1–3, respectively.
Acute and delayed vomiting CR rates (89%–91% and 84%–89%, respectively) were significantly higher than nausea CR rates (59%–66% and 55%–59%, respectively), supporting the contention that nausea and vomiting are separate phenomena and should be treated differently. More patients reported impact of nausea (35%) than vomiting (13%) on QoL overall.3 Nausea, which has been associated with anorexia, may be better controlled by medication classes other than antiemetics or nonpharmacological interventions. Patients who reported nausea did not consistently use rescue antiemetics, emphasizing that nausea is subjectively measured and a separate phenomenon than vomiting. Historically, the literature has combined the constructs of nausea and vomiting into one end point. Future trial designs should separate the two symptoms, because current guideline antiemetics may not effectively ameliorate nausea.
While these results are clinically significant and meaningful, this study was terminated early due to low accrual rates, and did not have the opportunity to reach predefined statistical thresholds for evaluation of treatment effectiveness. The low accrual rate was a direct result of rapidly changing treatment regimens and the lack of effective standardized therapy for patients with recurrent MGs. Although attempts to increase accrual were made by including patients receiving additional moderately emetic irinotecan-treatment combinations, most patients were treated with other available (targeted) therapies during the study enrollment period, which decreased accrual rates. Nevertheless, irinotecan and BEV remain two of the primary US Food and Drug Administration-approved drugs for recurrent glioma treatment, and supportive-care data for this regimen are essential. Therefore, despite its premature termination, the descriptive results of this study have important clinical implications for the recurrent glioma population.
The PAL–DEX regimen was well tolerated: few patients experienced PAL–DEX-related adverse events (12%). Few patients experienced the expected side effects (mild headache 2%, diarrhea 5%, constipation 5%). There were no reported grade 3 toxicities related to the biweekly PAL–DEX antiemetic regimen. More importantly, despite the well-documented association of 5-HT3-RAs with prolonged QT intervals, there were no cardiac toxicities with this regimen.
Because most guidelines are based on research utilizing single-dose chemotherapy regimens, there are no clear current guidelines for CINV management in patients receiving multiple-dose regimens. In this study, overall CR rates for multiple doses decreased over time. Overall CR rate for all three doses over a 6-week period was only 38% (Table 4), a suboptimal outcome, although overall CR rates over multiple doses were not an end point in this study. One contributing factor to reduction in CR rates over time was patient dropout due to progressive disease (not CINV), which reduced subject numbers and decreased the precision of estimates. Although patient dropout remains a challenge in research on patients receiving multidose chemotherapy, preventing CINV in this population should be a primary focus of future NV research.
Limitations
Limitations of this study included a nonrandomized design and lack of a control group. Due to rapidly changing treatment options for patients with recurrent glioma, for whom there was no consensus standardized treatment, accrual was incomplete, and study results were thus inconclusive within the framework of the original statistical design. Additionally, overall CR rates after doses 2 and 3 should be cautiously interpreted, because fewer patient surveys were completed and returned (primarily due to disease progression). If patients who dropped out experienced more or less CINV than those who completed the study, response bias could be an additional limitation.
Conclusion
The magnitude of response observed in the Phase II trial is consistent with the current evidence-based guideline literature, which identifies PAL plus DEX as an effective, well-tolerated antiemetic regimen for glioma patients receiving MEC. Antiemetic research on recurrent glioma patients receiving multidose, nonstandardized regimens is challenging. However, the data reported here support recommendations that additional PAL research should be conducted using alternative trial designs to accommodate small patient numbers, and Phase III randomized trials should be initiated when a standard regimen for recurrent glioma is established.
Acknowledgments
This work was supported by Eisai Inc (Duke Institutional Review Board Protocol number Pro00002273). The authors thank Elizabeth Flint and Wendy Gentry for their contributions in editing and the patients and staff at Preston Robert Tisch Brain Tumor Center, Duke Cancer Institute.
Disclosure
MLA and KBP have received research funding from Eisai. KBP also received research funding from Agios, Genentech, Merck, and VBL. MLA has participated as an advisory board member for Eisai and NEPA. KBP has participated as an advisory board member for Novocure and Agios. AD has participated as an advisory board member for Genentech/Roche, Cavion, Novella, EMD Serono, and PTC Therapeutics. HSF has participated as an advisory board member for Tactical Therapeutics. HSF has served as an advisor/speaker/consultant for Genentech. AD has stock/ownership interest with IST GmbH. AD and HSF hold letters of patent for oncolytic poliovirus human tumors. The other authors report no conflicts of interest in this work.
References
Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13(4):219–227. | ||
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting: results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97(12):3090–3098. | ||
Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374(14):1356–1367. | ||
Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482–2494. | ||
Hawkins R, Grunberg S. Chemotherapy-induced nausea and vomiting: challenges and opportunities for improved patient outcomes. Clin J Oncol Nurs. 2009;13(1):54–64. | ||
Chung SK, Ahn MJ, Yoo JY, et al. Implementation of best practice for chemotherapy-induced nausea and vomiting in an acute care setting. Int J Evid Based Healthc. 2011;9(1):32–38. | ||
Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100(10):2261–2268. | ||
Boccia RV. Chemotherapy-induced nausea and vomiting: identifying and addressing unmet needs. J Clin Outcomes Manag. 2013;20(8):377–384. | ||
Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. J Clin Oncol. 1999;17(9):2971–2994. | ||
Kris MG, Hesketh PJ, Herrstedt J, et al. Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer. 2005;13(2):85–96. | ||
Herrstedt J, Rapoport B, Warr D, et al. Acute emesis: moderately emetogenic chemotherapy. Support Care Cancer. 2011;19 Suppl 1:S15–S23. | ||
Wickham R. Best practice management of CINV in oncology patients: II. Antiemetic guidelines and rationale for use. J Support Oncol. 2010;8(2 Suppl 1):10–15. | ||
Likun Z, Xiang J, Yi B, Xin D, Tao ZL. A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist. 2011;16(2):207–216. | ||
Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer. 2011;19(6):823–832. | ||
Schwartzberg L, Barbour SY, Morrow GR, Ballinari G, Thorn MD, Cox D. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer. 2014;22(2):469–477. | ||
Kim JS, Kim JY, Lee SJ, et al. Multicenter nonrandomized trial of ramosetron versus palonosetron in controlling chemotherapy-induced nausea and vomiting for colorectal cancer. Ann Surg Treat Res. 2014;87(1):9–13. | ||
Navari RM. Palonosetron: a second generation 5-hydroxytryptamine 3 receptor antagonist. Expert Opin Drug Metab Toxicol. 2009;5(12):1577–1586. | ||
Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107(2):469–478. | ||
Rojas C, Li Y, Zhang J, et al. The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010;335(2):362–368. | ||
Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010;626(2–3):193–199. | ||
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Antiemesis. Version 1. Fort Washington (PA): NCCN; 2015. | ||
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Somerfield MR, Lyman GH. Antiemetic use in oncology: updated guideline recommendations from ASCO. Am Soc Clin Oncol Educ Book. 2012:532–540. | ||
Rozzi A, Nardoni C, Corona M, et al. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting in glioblastoma patients treated with temozolomide: a phase II study. Support Care Cancer. 2011;19(5):697–701. | ||
Affronti ML, Brickhouse A, Marcello J, et al. A phase II single arm trial of palonosetron (PALO) for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) in malignant glioma (MG) patients receiving irinotecan in combination with bevacizumab. Neuro Oncol. 2009;11(5):656. | ||
Feinberg B, Gilmore J, Haislip S, et al. Impact of initiating antiemetic prophylaxis with palonosetron versus ondansetron on risk of uncontrolled chemotherapy-induced nausea and vomiting in patients with lung cancer receiving multi-day chemotherapy. Support Care Cancer. 2012;20(3):615–623. | ||
Decker GM, DeMeyer ES, Kisko DL. Measuring the maintenance of daily life activities using the functional living index-emesis (FLIE) in patients receiving moderately emetogenic chemotherapy. J Support Oncol. 2006;4(1):35–41, 52. | ||
O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. | ||
Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda (MD): CTEP; 2006. | ||
Brearley SG, Clements CV, Molassiotis A. A review of patient self-report tools for chemotherapy-induced nausea and vomiting. Support Care Cancer. 2008;16(11):1213–1229. | ||
Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003;11(8):522–527. | ||
Martin AR, Carides AD, Pearson JD, et al. Functional relevance of antiemetic control: experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. Eur J Cancer. 2003;39(10):1395–1401. | ||
Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561. | ||
Martin CG, Rubenstein EB, Elting LS, Kim YJ, Osoba D. Measuring chemotherapy-induced nausea and emesis. Cancer. 2003;98(3):645–655. | ||
Ballatori E, Roila F. Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual Life Outcomes. 2003;1:46. | ||
Affronti ML, Schneider SM, Herndon JE 2nd, Schlundt S, Friedman HS. Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer. 2014;22(7):1897–1905. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.