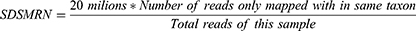Back to Journals » Infection and Drug Resistance » Volume 15
A Paired Comparison of Plasma and Bronchoalveolar Lavage Fluid for Metagenomic Next-Generation Sequencing in Critically Ill Patients with Suspected Severe Pneumonia
Authors Sun T, Liu Y, Cai Y, Zhai T, Zhou Y, Yang B, Wu X, Zhan Q
Received 16 May 2022
Accepted for publication 3 August 2022
Published 9 August 2022 Volume 2022:15 Pages 4369—4379
DOI https://doi.org/10.2147/IDR.S374906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Ting Sun,1 Yijie Liu,2 Ying Cai,3 Tianshu Zhai,3 Yun Zhou,4 Bin Yang,5 Xiaojing Wu,3,* Qingyuan Zhan1,3,*
1Capital Medical University China-Japan Friendship School of Clinical Medicine, Beijing, People’s Republic of China; 2Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 3Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, National Center for Respiratory Medicine, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 4Laboratory Medicine, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 5Vision Medicals Center for Infection Diseases, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingyuan Zhan, Capital Medical University China-Japan Friendship School of Clinical Medicine, No. 2 Yinghua East Road, Chaoyang District, Beijing, People’s Republic of China, Tel +86 13911785957, Fax +86 010-64217749, Email [email protected] Xiaojing Wu, Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, National Center for Respiratory Medicine, China-Japan Friendship Hospital, No. 2 Yinghua East Road, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +86 13811743537, Fax +86 010-64217749, Email [email protected]
Purpose: Plasma metagenomic next-generation sequencing (mNGS) has emerged as an attractive and minimally invasive technique for pathogen detection. However, few studies have demonstrated the need for simultaneous plasma and bronchoalveolar lavage fluid (BALF) mNGS in patients with severe pneumonia.
Patients and Methods: This study retrospectively performed a paired comparison of BALF and plasma mNGS in critically ill patients with suspected severe pneumonia from April 2019 to December 2020. The diagnostic performance of BALF and plasma mNGS was compared using the clinical composite diagnosis as the reference standard.
Results: In total, 57 patients were included in this study. Patients with positive plasma mNGS had shorter hospital stay days at the time of specimen acquisition (4.5 vs 11, P = 0.028) and a higher positivity rate of BALF culture (50% vs 22.9%, P = 0.033) than patients with negative plasma mNGS. Fifty-three patients (93%) were finally diagnosed with severe pneumonia. Significant differences were observed in the sensitivity of BALF and plasma mNGS (100% vs 42%, P < 0.001), and the diagnostic accuracy was 96% and 46%, respectively. The proportion of virus in positive plasma mNGS results was higher than that in BALF mNGS (23% vs 11%, P = 0.173) without significant difference. Although plasma mNGS detected additional microorganisms in 11/53 patients, the beneficial effect was observed in only 5/53 (9%) patients.
Conclusion: In this study, the clinical effect of simultaneously conducting mNGS of BALF and plasma samples was found to be limited. For patients with the suspected virus infection, plasma mNGS may be a supplementary test. Further studies are needed to identify the optimal indications for plasma mNGS.
Keywords: mNGS, infection, cell-free DNA, diagnosis, pathogen
Introduction
Severe pneumonia is one of the most common causes of infectious diseases among patients in the intensive care unit (ICU), and this can lead to various complications and high mortality.1–3 Timely and accurate pathogen diagnoses are crucial for appropriate antimicrobial therapy and improved clinical outcomes. However, the low detection rate and time-consuming process (3–5 days) of conventional methods, including culture and serological tests, are the problems faced by clinicians.4 Pathogens have been detected in only 38% of patients with radiographic evidence of pneumonia.1 In a study reported by Leven et al,5 even the combination of culture-based identification, targeted molecular methods, and serology tests, yielded the potential pathogen detection rate of only 59% in patients with lower respiratory tract (LRT) infection. For critically ill patients with severe pneumonia, the complexities of illness and prior antibiotic exposure have further complicated the effective detection of pathogens.
Metagenomic next-generation sequencing (mNGS) is a potential diagnostic tool for severe pneumonia6–9 because of its broad range of detection and a shorter turn around time than culture methods.10 Invasive clinical specimens, such as LRT aspirates, bronchoalveolar lavage fluid (BALF), bronchial needle brushing, and even lung biopsy tissues,7,11–13 are generally necessary to perform mNGS for patients with severe pneumonia. In recent years, plasma mNGS has been developed to diagnose a wide range of clinical infectious diseases14–17 and is expected to be useful for predicting impending bloodstream infections to guide pre-emptive therapy.18 Unlike BALF, the plasma sample is more accessible and hence, the procedure is less invasive. However, clinical studies investigating the performance of plasma mNGS in patients with severe pneumonia are limited and controversial.19–23 Studies have reported that plasma mNGS has moderate sensitivity and high specificity for invasive mold infections in pulmonary patients23 and is highly consistent with BALF mNGS in patients with severe pneumonia.19 Yang et al24 also found a novel link between plasma mNGS and systemic inflammation in patients with severe pneumonia. However, two other studies suggested that BALF mNGS is more sensitive than plasma mNGS in patients with pneumonia, and concordance between paired BALF and plasma was not sufficient.21,22 Notably, for critically ill patients with pneumonia, antibiotic administration before sampling is an unavoidable and complex bias for the mNGS assessment. One study suggested that although plasma is a poorer indicator than the respiratory sample, plasma mNGS could be an alternative for detecting pneumonia pathogens when respiratory specimens are unavailable.25 The necessity of simultaneously testing both BALF and blood samples thus remains unclear.
This study aimed to evaluate the clinical diagnostic utility of simultaneous plasma and BALF samples using mNGS in patients with severe pneumonia and determine the difference between the two samples for the mNGS test in clinical practice.
Materials and Methods
Study Design and Specimen Processing
All patients with suspected severe pneumonia admitted to the medical ICU at China–Japan Friendship Hospital from April 2019 to December 2020 were retrospectively investigated. Patients with suspected pneumonia, including community-acquired pneumonia26 and hospital-acquired pneumonia,27 were enrolled after meeting the following inclusion criteria: older than 18 years, meeting the severe pneumonia diagnostic criteria of the Infectious Diseases Society of America/American Thoracic Society consensus guidelines,28 and with available BALF and plasma mNGS results. Patients were excluded if the BALF and plasma were not acquired simultaneously for the mNGS or the patient had been discharged before the mNGS results were obtained. Septic shock29 and immunosuppression were defined according to a previous study.30 For all enrolled patients, demographic and clinical data were collected, including comorbidities, laboratory test results, microbiological testing, days of hospital/ICU stay at the time of specimen acquisition, Median Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, ICU treatment data, and patient outcomes.
This study was approved by the Institutional Review Board of the China–-Japan Friendship Hospital (2019–180-K121-1) and performed in accordance with the Declaration of Helsinki. The need for informed consent was waived due to the retrospective nature of the study and because the data were anonymously analyzed.
Conventional Microbiological Tests
The conventional microbiological tests (CMTs) included bacterial or fungal smear and culture, acid-fast stain, and real-time polymerase chain reaction (PCR) to detect cytomegalovirus (CMV), Epstein–Barr virus (EBV), influenza virus, respiratory syncytial virus, Pneumocystis jirovecii, Legionella, Mycoplasma, and Chlamydia. PCR was performed at the clinician’s discretion. Previous studies have reported that the CMTs of BALF samples (smear, culture, and PCR) were consistent with the standard clinical procedure.31,32
mNGS
DNA sequencing of both the BALF and plasma samples was conducted for each patient, and RNA sequencing of BALF libraries was conducted only for patients with a suspected viral infection. BALF DNA was extracted from all samples using the QIAamp® UCP Pathogen DNA Kit (Qiagen) following the manufacturer’s instructions. Human DNA was removed using Benzonase (Qiagen) and Tween 20 (Sigma). BALF total RNA was extracted using the QIAamp® Viral RNA Kit (Qiagen), and ribosomal RNA was removed using the Ribo-Zero rRNA Removal Kit (Illumina, San Diego, CA, USA). Cell-free DNA in plasma was extracted using the QIAamp® Circulating Nucleic Acid Kit (Qiagen). Extracted DNA was used to generate libraries using the Nextera XT kit (Illumina) according to the manufacturer’s instructions. The library was sequenced on an Illumina NextSeq 550 sequencer using a 75 bp sequencing strategy. For negative controls, we also prepared peripheral blood mononuclear cell samples with 105 cells/mL from healthy donors in parallel with each batch using the same protocol, and sterile deionized water was extracted alongside the specimens to serve as non-template controls.
Fastp was used to remove low-quality reads, adapter contamination, duplicate reads, and those shorter than 50 bp. Low-complexity reads were removed using Kcomplexity with default parameters.33 The human sequence data were identified and excluded by mapping to a human reference genome (hg19 and hg38) using SNAP v1.0beta.18.34 To construct the microbial genome database, pathogens and their genomes or assemblies were selected following the Kraken 2 criteria for selecting representative assemblies for microorganisms (bacteria, viruses, fungi, protozoa, and other multicellular eukaryotic pathogens) from an open-access Kraken2 database (https://benlangmead.github.io/aws-indexes/k2). Microbial reads were aligned to the database using Burrows–Wheeler Aligner software.35 We defined reads with fewer than four mismatches of reference as “mapped reads.” Reads with multiple locus alignments within the same genus were excluded in secondary analysis. Only reads mapped to the genome within the same species were considered. We defined a bioinformatic parameter, standardized strictly mapped reads number (SDSMRN), using the formula below:
Pathogen and Clinical Composite Diagnosis
Given the lack of standard threshold criteria for interpreting mNGS results, we used the criteria derived and revised from previous studies.36–40 The mNGS results were considered as positive if one of the following thresholds were met, and a literature evidence of its pulmonary pathogenicity is available: (i) culture/PCR and mNGS identified the same microbe; (ii) at the species level, the relative abundance of bacteria (mycobacteria excluded), fungi (molds excluded), and parasites was greater than 30%, or the coverage rate scored 10-fold greater than that of any other microbes (for fungi, whose coverage rate scored 5-fold higher than that of any other fungus); (iii) positive virus and molds will be considered when the SDSMRN was no less than 3; (iv) for the detection of Mycobacterium spp., Nocardia spp., the pathogen was considered as positive when SDSMRN is greater than 1.
Microorganisms with unclear significance, such as Torque teno virus (TTV) and EBV, were considered “non-pathogenic microbes.” Oral and intestinal microorganisms were identified as colonized or contaminated unless clinical evidence is available, such as aspiration. All the mNGS results were classified as “probable pathogen”, “definite pathogen”, or “non-pathogenic microbe.” Only the microbe that was defined as “probable pathogen” or “definite pathogen” was considered as a true positive mNGS result.
To confirm the clinical composite diagnosis, two experienced infectious disease specialists who were experienced in the ICU independently reviewed the medical records of each patient. First, they determined whether the patients had infectious pneumonia; next, they identified the clinical composite diagnosis based on clinical manifestation, laboratory tests, and chest radiology, microbiological tests (including CMTs and mNGS).39,41
The impact of mNGS results was assessed according to the data obtained. The clinical impact was classified as no change and treatment change, wherein the initial antimicrobial drugs were added or reduced according to the mNGS results.
Statistical Analysis
The t-test and Wilcoxon rank test were used for the comparative analysis of the continuous variables. Pearson chi-square, Fisher exact, or McNemar tests were used for discrete variables where appropriate. For the sensitivity, specificity, positive predictive value, and negative predictive value, we used the clinical composite diagnosis as the reference standard. Data analyses were performed using the SPSS 26.0 (IBM, Armonk, NY, USA) software and Empower (R) (X&Y solutions, Inc. Boston, MA, USA). P values < 0.05 were considered significant, and all tests were two-tailed.
Results
Patient Characteristics
During the study period, 24 of 81 ICU patients with suspected severe pneumonia who had both BALF and plasma mNGS results were excluded; this was done either because BALF and plasma mNGS were not performed simultaneously or because the patient had been discharged before the mNGS results were obtained. In total, 57 patients (median age: 59.9 years, male: 40, female: 17) were enrolled in this study and were divided into the negative plasma mNGS (n = 35) and positive plasma mNGS (n = 22) groups. The baseline characteristics and outcomes of patients are shown in Table 1. 29/57 (50.9%) received mechanical ventilation. Seventeen of the 57 patients were diagnosed with septic shock, and 31 had immunocompromised conditions. The median APACHE II and SOFA scores were 14.5 and 4, respectively. Days of hospital and ICU stay at the time of specimen acquisition were 7 and 2 d, respectively. Additionally, 49/57 (86%) of the patients had been treated with antibiotics before sampling, and 19 (36%) patients had a positive BALF culture. Patients with positive plasma mNGS had fewer days of hospital stay at the time of specimen acquisition (4.5 vs 11, P = 0.028), had higher positive BALF culture rates (50% vs 22.9%, P = 0.033), and were younger (52.1 vs 64.7, P = 0.001), compared with the patients with negative plasma mNGS. No significant differences in the severity of illness (APACHE II, SOFA, and septic shock) and total 28-day mortality were observed between the negative and positive plasma mNGS groups (Table 1).
 |
Table 1 Characteristics of Patients with Suspected Severe Pneumonia Included in the Study |
Comparison of mNGS Results Between BALF and Plasma
In total, 53/57 patients (93%) were diagnosed with severe pneumonia, and the remaining four were diagnosed with idiopathic interstitial pneumonia (n = 3) and secondary organizing pneumonia (n = 1). Fifty-three (100%) true positive results were obtained using BALF mNGS from all 53 patients with severe pneumonia, and two of the four patients with a non-infectious etiology had negative BALF mNGS results. However, 22/53 (41%) of patients with severe pneumonia had true positive plasma mNGS results. The most frequently detected pathogens using BALF mNGS were Pneumocystis jirovecii, Pseudomonas aeruginosa, and Klebsiella pneumonia (Figure 1 and Supplementary Data Table S1). Meanwhile, CMV, P. jirovecii, and K. pneumonia were the most common pathogens observed in plasma mNGS results (Figure 1). All the results of microbiological tests are shown in Supplementary data Table S1.
 |
Figure 1 Pathogen spectrum of mNGS results in patients with severe pneumonia (n = 53). Abbreviations: BALF, bronchoalveolar lavage fluid; mNGS, metagenomic next-generation sequencing. |
Among the 53 patients with severe pneumonia, significant differences in the positivity rate of BALF and plasma mNGS were observed (100% vs 41%, P < 0.001; Figure 2A). In 27/53 cases, mNGS identified two or more organisms (polymicrobial infections) without difference in the polymicrobial infection rate between the positive mNGS results of BALF and plasma [25/53 (47%) vs 8/22 (15%), P = 0.479; Figure 2A). In total, 94 and 32 pathogens were detected using BALF and plasma mNGS, respectively (Figure 2B). In 13 cases, more than two co-infecting pathogens were detected using BALF mNGS, with only one such case detected using plasma mNGS (Figure 2C). Although no significant difference was observed, the proportion of bacteria detected using BALF mNGS was higher than that using plasma mNGS (42% vs 32%, P = 0.301; Figure 2D). However, the proportion of virus in positive plasma mNGS results was higher than in BALF mNGS (23% vs.11%, P = 0.173; Figure 2D).
Diagnostic Performance of BALF and Plasma mNGS
In total, 28/53 patients had positive CMT results, including 19 cases with a positive BALF culture test. No patient had a positive blood culture test. As shown in Table 2, based on the clinical composite diagnosis as a reference, plasma mNGS had a much lower sensitivity (100% vs 42%, P < 0.001), and the diagnostic accuracies of mNGS using BALF and plasma samples were 96% and 46%, respectively. However, in this study, the specificity of plasma mNGS was 100%, whereas that of BALF mNGS was 50%. Among 28 patients with positive CMT results, the positivity rate of plasma mNGS was 43% (12/28). The Kappa value of the two methods was 0.37 (0.12–0.60). The sensitivity of a single plasma mNGS test was not superior to that of CMT (42% vs 53%).
 |
Table 2 Diagnostic Performance of mNGS and CMTs in Patients with Suspected Severe Pneumonia (n = 57) |
Clinical Impact of Plasma mNGS
Plasma mNGS reported an additional 12 microorganisms in 11 patients, including EBV, CMV, TTV, human alphaherpesvirus 3, Aspergillus, and human adenovirus, as shown in Table 3. Specifically, four pathogens were defined as definite, three were probable, and five microorganisms were unlikely, based on ICU clinical composite adjudication. In this study, the beneficial effects were categorized as a “treatment change” relative to the initial treatment. Based on the additional organisms that were identified using plasma mNGS, a beneficial impact was obtained for 5/53 (9%) patients (Table 3). A multicenter retrospective study showed that the positive clinical impact of plasma mNGS for the diagnosis of infections is 7.3%.16
 |
Table 3 Impact of Microorganisms Detected Using Only Plasma mNGS in the Paired Sample (11 Cases) |
Examples of cases in which plasma mNGS resulted in an antimicrobial change were as follows: 1) for patient no. 6, a 46-year-old immunocompromised patient with severe pneumonia, voriconazole was administered when Aspergillus was detected using only plasma mNGS, whereas the BALF mNGS and CMT detected Nocardia cyriacigeorgica, P. jirovecii, and CMV; 2) for patient nos. 43 and 55, CMV was identified using only plasma mNGS, and thus ganciclovir was added to the treatment regimen; and 3) for patient nos. 41 and 53, who were diagnosed with human alphaherpesvirus 3 infection based on the plasma mNGS, were treated with acyclovir.
Discussion
This study conducted a paired comparison between BALF and plasma mNGS in terms of their performance in detecting pathogens in ICU patients with suspected severe pneumonia. The reliance on BALF mNGS, but not plasma mNGS, to identify pathogens in patients with severe pneumonia is supported by the results of this study. Plasma mNGS has recently been regarded as a promising and less invasive microbial detection technique for severe pneumonia. A previous study found that the release of pathogen DNA into the bloodstream might be more common than appreciated in pneumonia patients with negative blood cultures,25 and that the DNA burden in body fluids is 160-fold higher than that in plasma.42 Additionally, the cost of one sample subjected to mNGS is approximately $400; therefore, the cost of using both BALF and plasma mNGS for patients with severe pneumonia must be considered.
In this study, BALF mNGS was superior for pathogen detection than plasma mNGS, and the diagnostic accuracy was higher than that of plasma mNGS (96% vs 46%) in patients with severe pneumonia. Hill et al23 also found that plasma mNGS had moderate sensitivity and high specificity for detecting pathogenic molds in hematopoietic cell transplant recipients with pneumonia.
Current knowledge indicates that the microorganisms in plasma might be transmitted from the lungs to the bloodstream of patients. However, in this study, the distribution of detected pathogenic species obviously differed between the results of BALF and plasma mNGS, and only 15/57 (26%) patients with suspected pneumonia had at least one plasma mNGS result that matched that of the BALF mNGS. To the best of our knowledge, only four studies have evaluated the clinical significance of simultaneous BALF and plasma mNGS in patients with pneumonia, but these studies do not have a consistent conclusion. Chen et al19 reported that the results of plasma mNGS were highly consistent with those of BALF samples. Jiang et al20 only evaluate the utility of plasma mNGS for pneumocystis jirovecii pneumonia. Xie et al21 presented a description of the results of both samples in 37 patients. The other remaining study used the results from the culture method, which did not use CMTs as the reference standard, and was consistent with our results, in which BALF mNGS was more sensitive than plasma mNGS.22
Furthermore, we found that the patients with positive plasma mNGS had shorter days of hospital stay at the time of specimen acquisition and a higher positivity rate of BALF culture than those with negative plasma mNGS. These results infer that the sensitivity of plasma mNGS could be influenced by the number of days of hospital stay at specimen acquisition. Future studies using a larger cohort should be performed to assess the generalizability of these findings.
We can detect new microorganisms that were previously unidentified members of the human microbiome.43 However, blood samples contain molecules originating from virtually every tissue, including the microorganisms that colonize the body. In this study, viruses were the most detected pathogen (12/32) in all plasma mNGS results, whereas bacteria (50/94) were most detected in BALF mNGS. This finding is consistent with the study of Chen et al,22 in which plasma mNGS was found to have advantages in identifying viruses. Fang et al44 reported that the plasma mNGS also can be clinically useful for treatment monitoring in patients with severe pneumonia and viremia. Notably, microorganisms, including TTV, EBV, and CMV, were only detected using plasma mNGS and not using BALF mNGS in 11/57 patients. Although plasma mNGS detected additional viral pathogens, these results had no clinical impact. Nevertheless, a beneficial impact was obtained in 45.5% (5/11) of the patients. Further studies should be conducted to determine if the microorganisms detected using only plasma mNGS, especially viruses, are derived from pneumonia or only reflect uncultivable microorganisms present in the blood. A previous study reported that anelloviruses (including TTV) could be identified in 96% of adult human blood samples during steroid-refractory/dependent graft-versus host disease.17 The clinical significance of these viruses is poorly understood; however, anelloviruses were reported as non-pathogenic endogenous markers of the immune function,45 and the quantification of TTV can guide immunosuppression to reduce graft rejection and infection.46 We suggest that routine and simultaneous BALF and plasma mNGS is unnecessary, as doing so will not be more beneficial to patients and only increase the hospital expenses. For patients with suspected virus infection, the paired plasma mNGS may be helpful.
This study had some limitations. First, this was a single-center retrospective study with a small cohort. We mostly included patients with prior antibiotic exposure, and some patients had an average of 7 d of hospital stay at the time of specimen acquisition, which might have affected the analysis results. Prior antibiotic exposure is an inevitable question in patients admitted to the ICU with suspected severe pneumonia. We used a paired comparison of simultaneous plasma and BALF samples of the same patient to reduce this bias. Second, the mNGS results from plasma did not include RNA mNGS tests. Third, although we did not perform specific screening, all patients had negative blood cultures, and thus, all positive cultures were derived from BALF samples, and the impact of plasma mNGS could not be further evaluated in patients with bloodstream infections.
Conclusion
Based on the results of our study, we do not recommend performing BALF and plasma mNGS simultaneously. For patients with suspected virus infection, plasma mNGS can be a supplementary test. Prospective studies are required to clarify the optimal indications for plasma mNGS as an additional test for patients with severe pneumonia.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article. The datasets generated during the current study are available in the NCBI repository, https://www.ncbi.nlm.nih.gov;BioProject: PRJNA729853.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81870072], the horizontal subject of China-Japan Friendship Hospital [grant number 2019-HX-77], the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [grant number 2022-I2M-JB-016], and the National High Level Hospital Clinical Research Funding [grant number 2022-NHLHCRF-LX-01].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi:10.1056/NEJMoa1500245
2. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi:10.1056/NEJMoa1306801
3. Laporte L, Hermetet C, Jouan Y, et al. Ten-year trends in intensive care admissions for respiratory infections in the elderly. Ann Intensive Care. 2018;8(1):84. doi:10.1186/s13613-018-0430-6
4. Ferreira-Coimbra J, Sarda C, Rello J. Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther. 2020;37(4):1302–1318. doi:10.1007/s12325-020-01248-7
5. Ieven M, Coenen S, Loens K, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24(11):1158–1163. doi:10.1016/j.cmi.2018.02.004
6. Xie Y, Du J, Jin W, et al. Next generation sequencing for diagnosis of severe pneumonia: china, 2010–2018. J Infect. 2019;78(2):158–169. doi:10.1016/j.jinf.2018.09.004
7. Li Y, Sun B, Tang X, et al. Application of metagenomic next-generation sequencing for bronchoalveolar lavage diagnostics in critically ill patients. Eur J Clin Microbiol Infect Dis. 2020;39(2):369–374. doi:10.1007/s10096-019-03734-5
8. Chen Y, Feng W, Ye K, et al. Application of metagenomic next-generation sequencing in the diagnosis of pulmonary infectious pathogens from bronchoalveolar lavage samples. Front Cell Infect Microbiol. 2021;11:541092. doi:10.3389/fcimb.2021.541092
9. Zhou H, Larkin PMK, Zhao D, et al. Clinical impact of metagenomic next-generation sequencing of bronchoalveolar lavage in the diagnosis and management of pneumonia: a multicenter prospective observational study. J Mol Diagn. 2021;23(10):1259–1268. doi:10.1016/j.jmoldx.2021.06.007
10. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
11. Kalantar KL, Moazed F, Christenson SC, et al. Metagenomic comparison of tracheal aspirate and mini-bronchial alveolar lavage for assessment of respiratory microbiota. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L578–L584. doi:10.1152/ajplung.00476.2018
12. Azar MM, Schlaberg R, Malinis MF, et al. Added diagnostic utility of clinical metagenomics for the diagnosis of pneumonia in immunocompromised adults. Chest. 2020. doi:10.1016/j.chest.2020.11.008
13. Wang Q, Wu B, Yang D, et al. Optimal specimen type for accurate diagnosis of infectious peripheral pulmonary lesions by mNGS. BMC Pulm Med. 2020;20(1):268. doi:10.1186/s12890-020-01298-1
14. Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–674. doi:10.1038/s41564-018-0349-6
15. Benamu E, Gajurel K, Anderson JN, et al. Plasma microbial cell-free DNA next generation sequencing in the diagnosis and management of febrile neutropenia. Clin Infect Dis. 2021. doi:10.1093/cid/ciab324
16. Hogan CA, Yang S, Garner OB, et al. Clinical impact of metagenomic next-generation sequencing of plasma cell-free DNA for the diagnosis of infectious diseases: a multicenter retrospective cohort study. Clin Infect Dis. 2021;72(2):239–245. doi:10.1093/cid/ciaa035
17. Zanella MC, Cordey S, Laubscher F, et al. Unmasking viral sequences by metagenomic next-generation sequencing in adult human blood samples during steroid-refractory/dependent graft-versus-host disease. Microbiome. 2021;9(1):28. doi:10.1186/s40168-020-00953-3
18. Goggin KP, Gonzalez-Pena V, Inaba Y, et al. Evaluation of plasma microbial cell-free DNA sequencing to predict bloodstream infection in pediatric patients with relapsed or refractory cancer. JAMA Oncol. 2020;6(4):552–556. doi:10.1001/jamaoncol.2019.4120
19. Chen J, Zhao Y, Shang Y, et al. The clinical significance of simultaneous detection of pathogens from bronchoalveolar lavage fluid and blood samples by metagenomic next-generation sequencing in patients with severe pneumonia. J Med Microbiol. 2021;70(1). doi:10.1099/jmm.0.001259
20. Jiang J, Bai L, Yang W, et al. Metagenomic next-generation sequencing for the diagnosis of pneumocystis jirovecii pneumonia in non-HIV-infected patients: a retrospective study. Infect Dis Ther. 2021;10(3):1733–1745. doi:10.1007/s40121-021-00482-y
21. Xie F, Duan Z, Zeng W, et al. Clinical metagenomics assessments improve diagnosis and outcomes in community-acquired pneumonia. BMC Infect Dis. 2021;21(1):352. doi:10.1186/s12879-021-06039-1
22. Chen X, Ding S, Lei C, et al. Blood and bronchoalveolar lavage fluid metagenomic next-generation sequencing in pneumonia. Can J Infect Dis Med Microbiol. 2020;2020:6839103. doi:10.1155/2020/6839103
23. Hill JA, Dalai SC, Hong DK, et al. Liquid biopsy for invasive mold infections in hematopoietic cell transplant recipients with pneumonia through next-generation sequencing of microbial cell-free DNA in plasma. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1639
24. Yang H, Haidar G, Al-Yousif NS, et al. Circulating microbial cell-free DNA is associated with inflammatory host-responses in severe pneumonia. Thorax. 2021;76(12):1231–1235. doi:10.1136/thoraxjnl-2020-216013
25. Langelier C, Fung M, Caldera S, et al. Detection of pneumonia pathogens from plasma cell-free DNA. Am J Respir Crit Care Med. 2020;201(4):491–495. doi:10.1164/rccm.201904-0905LE
26. Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. 2018;12(4):1320–1360. doi:10.1111/crj.12674
27. Shi Y, Huang Y, Zhang TT, et al. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition). J Thorac Dis. 2019;11(6):2581–2616. doi:10.21037/jtd.2019.06.09
28. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
29. Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):775–787. doi:10.1001/jama.2016.0289
30. Ramirez JA, Musher DM, Evans SE, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. 2020;158(5):1896–1911. doi:10.1016/j.chest.2020.05.598
31. Zhou F, Wang Y, Liu Y, et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China Network. Eur Respir J. 2019;54(2). doi:10.1183/13993003.02406-2018
32. Mu S, Hu L, Zhang Y, et al. Prospective evaluation of a rapid clinical metagenomics test for bacterial pneumonia. Front Cell Infect Microbiol. 2021;11:684965. doi:10.3389/fcimb.2021.684965
33. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi:10.1093/bioinformatics/btu170
34. Zaharia M, Bolosky WJ, Curtis K, et al. Faster and more accurate sequence alignment with SNAP; 2011. Available from: https://arxiv.org/abs/1111.5572.
35. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324
36. Li H, Gao H, Meng H, et al. Detection of pulmonary infectious pathogens from lung biopsy tissues by metagenomic next-generation sequencing. Front Cell Infect Microbiol. 2018;8:205. doi:10.3389/fcimb.2018.00205
37. Qian YY, Wang HY, Zhou Y, et al. Improving pulmonary infection diagnosis with metagenomic next generation sequencing. Front Cell Infect Microbiol. 2020;10:567615. doi:10.3389/fcimb.2020.567615
38. Zhang F, Chen J, Huang H, et al. Application of metagenomic next-generation sequencing in the diagnosis and treatment guidance of Pneumocystis jirovecii pneumonia in renal transplant recipients. Eur J Clin Microbiol Infect Dis. 2021;40(9):1933–1942. doi:10.1007/s10096-021-04254-x
39. Peng JM, Du B, Qin HY, Wang Q, Shi Y. Metagenomic next-generation sequencing for the diagnosis of suspected pneumonia in immunocompromised patients. J Infect. 2021;82(4):22–27. doi:10.1016/j.jinf.2021.01.029
40. Qu J, Zhang J, Chen Y, et al. Aetiology of severe community acquired pneumonia in adults identified by combined detection methods: a multi-centre prospective study in China. Emerg Microbes Infect. 2022;11(1):556–566. doi:10.1080/22221751.2022.2035194
41. Zhan Y, Xu T, He F, et al. Clinical evaluation of a metagenomics-based assay for pneumonia management. Front Microbiol. 2021;12:751073. doi:10.3389/fmicb.2021.751073
42. Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27(1):115–124. doi:10.1038/s41591-020-1105-z
43. Kowarsky M, Camunas-Soler J, Kertesz M, et al. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc Natl Acad Sci U S A. 2017;114(36):9623–9628. doi:10.1073/pnas.1707009114
44. Fang X, Xu M, Fang Q, et al. Real-time utilization of metagenomic sequencing in the diagnosis and treatment monitoring of an invasive adenovirus B55 infection and subsequent herpes simplex virus encephalitis in an immunocompetent young adult. Open Forum Infect Dis. 2018;5(6):ofy114. doi:10.1093/ofid/ofy114
45. Rezahosseini O, Drabe CH, Sorensen SS, et al. Torque-Teno virus viral load as a potential endogenous marker of immune function in solid organ transplantation. Transplant Rev. 2019;33(3):137–144. doi:10.1016/j.trre.2019.03.004
46. Doberer K, Schiemann M, Strassl R, et al. Torque teno virus for risk stratification of graft rejection and infection in kidney transplant recipients-A prospective observational trial. Am J Transplant. 2020;20(8):2081–2090. doi:10.1111/ajt.15810
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.