Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
A novel water-based anti-aging suncare formulation provides multifaceted protection and repair against environmental aggressors: evidence from in vitro, ex vivo, and clinical studies
Authors Narda M , Ramos-Lopez D, Bustos J, Trullàs C , Granger C
Received 23 March 2019
Accepted for publication 20 June 2019
Published 23 July 2019 Volume 2019:12 Pages 533—544
DOI https://doi.org/10.2147/CCID.S209728
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Mridvika Narda, David Ramos-Lopez, Javier Bustos, Carles Trullàs, Corinne Granger
Innovation and Development, ISDIN SA, Barcelona, Spain
Background: Ultraviolet (UV) radiation is an established cause of skin aging, and the role of pollution is increasingly acknowledged. In this study, we evaluated the efficacy of an antipollution and anti-aging suncare product in in vitro, ex vivo, and clinical studies.
Methods: We assessed 1) sunburn cell (SBC) and cyclobutane pyrimidine dimer (CPD) formation and gene expression profile in reconstructed human epidermis following solar irradiation, 2) malondialdehyde (MDA) level, Nrf2 immunostaining, and genetic expression in skin explants exposed to pollution, 3) carbon particle adhesion to healthy forearm skin in a clinical study, and 4) skin firmness, elasticity, and pigmentation spots in healthy women following 56 days of application.
Results: 1) The product fully protected against CPD formation, and provided a high protection against SBC formation, with levels close to non-irradiated samples. Expression of genes encoding pro-inflammatory and oxidative stress response markers was lower in product-treated than untreated skin. 2) Compared with pollution-exposed untreated controls, product-treated skin had 23% lower MDA levels (P<0.01), weaker Nrf2 immunostaining, and attenuated upregulation of CYP1A1. 3) The product significantly decreased adhesion of carbon particles to the skin (15.2% less than control; P<0.01). 4) Clinically, product use led to a decrease in brown spots, with a relative reduction in the count of −1.9% (NS), and in area, −5.0% (P<0.01), and decrease in UV spots, with a relative reduction of −6.9% (P<0.01) and −9.3% (P=0.02) for count and area, respectively. Firmness increased significantly by 14.1% (P<0.01). Participants reported skin was more even in tone (80%), more moisturized (93%), and firmer (74%).
Conclusion: This water-based anti-aging SPF50 suncare formulation containing photolyase encapsulated in liposome, active biopeptides, antioxidants, and hyaluronic acid provides multifaceted protection and repair action against pollution and UV-induced skin aging, ideal for everyday use.
Keywords: photolyase, cyclobutane pyrimidine dimers, anti-aging, photoprotection, pollution
Introduction
Facial skin quality plays a crucial role in the perception of health and attractiveness. Yet the face is often the part of the body most exposed to the factors responsible for accelerated skin damage and aging. The susceptibility of skin to the harmful effects of ultraviolet (UV) A and B radiation is well established.1,2 Solar radiation is linked to epidermal changes characterized by loss of barrier function, scaling, and dryer skin,3 as well as dermal changes with degradation of extracellular matrix proteins due to activation of matrix metalloproteinases.4 This set of changes are collectively termed photoaging, and daily use of broad-spectrum sunscreen providing protection against UVB and UVA is recommended to prevent such damage. However, many users find compliance challenging, largely due to sunscreens frequently having unpleasantly oily or chalky textures, leaving a white residue or an oily sheen on the skin.
A growing body of evidence suggests that other factors such as air pollution,5–8 smoking, lack of sleep, stress, and diet also play a significant role in skin aging.9,10 Air pollution in particular has garnered much attention in both the scientific and lay press, as a mediator of oxidative stress within the skin, accelerating the appearance of signs of aging. Air pollution refers to ultrafine particles originating from automotive exhausts, industrial fumes, or agricultural smoke that remain suspended in the air along with toxic gases. On contact with the outermost layers of skin, these ultrafine particles, which can act as carriers for toxic chemicals,11 generate a series of reactions leading to production of reactive oxygen species. Externally, skin exposed to pollution daily, as might happen in polluted urban centers, develops wrinkles and hyperpigmentation, manifesting as dark spots or yellowish skin tone.11
For an aging population, the incorporation of anti-aging or rejuvenating products into daily skin care has become increasingly relevant. Such products play an important role in improving the general appearance and texture of facial skin through increasing skin hydration, and improving skin firmness and tone by combining a host of active cosmetic ingredients. For persons looking for sun protection, protection from pollution and anti-aging benefits, the use of multiple products daily that this implies can hinder compliance and thus reduce the benefit. In addition, such products may be incompatible with each other, leading to a poor aesthetic result. With this in mind, we sought to develop a product that could provide multifaceted protection against and repair of solar and pollution-induced skin damage.
An all-in-one, daily-use product was designed to prevent and repair skin damage resulting from exposure to solar radiation and pollution. The product offers a very high sun protection factor (SPF50) and UVA protection that supports its use as a daily-use sunscreen. For a more complete approach to solar skin protection, it also contains the DNA repair enzyme photolyase, derived from plankton extract, to complement the skin’s inherent DNA repair mechanisms. In addition, to provide protection against pollution and anti-aging or skin rejuvenation benefits, the product contains hyaluronic acid, palmitoyl tripeptide-38, and pentapeptide-34 trifluoroacetate. The product was developed to have an ultra-light water-based texture (oil-in-water base) with quick absorption that is compatible with use under makeup. We report here the in vitro, ex vivo, and clinical studies performed to assess the efficacy of this facial fluid in protecting against UV- and pollution-induced changes and improving visible signs of aging.
Materials and methods
Investigational product
The investigational product (IP) was a water-based SPF50 facial suncare formulation containing a combination of organic and inorganic UV filters (butyl methoxydibenzoylmethane, ethylhexyl methoxycinnamate, ethylhexyl triazone, and titanium dioxide), a potent combination of antioxidants (tocopheryl acetate, tocopherol, butylated hydroxytoluene, ascorbyl palmitate, ascorbic acid), a source of DNA repair enzyme photolyase (plankton extract) and pollutant protective and anti-aging ingredients (sodium hyaluronate, palmitoyl tripeptide-38, and pentapeptide-34 trifluoroacetate).
Reconstructed human epidermis (RHE) and UV exposure
A RHE model, established from normal human epidermal keratinocytes isolated from a single donor foreskin,12 was used to assess the effects of the product against UV irradiation. Ten-day-old RHE was cultured in maintenance medium at 37°C and 5% CO2, treated topically with the IP (5 mg/cm2) and incubated for 24 hrs. Product was reapplied just before skin was irradiated for approximately 45 mins (500 mJ/cm2 UVB and 5.1 J/cm2 UVA) with a SOL500 Sun Simulator (Dr Hönle AG, Munich, Germany). All experimental conditions were performed in duplicate and two parallel controls of untreated irradiated and non-irradiated epidermis were used.
Human ex vivo skin explants and pollution exposure
Skin was obtained from a consenting abdominoplasty donor, then divided and maintained in culture medium at 37°C/5% CO2 atmosphere. Skin explants were exposed to nebulized urban dust mixture as previously described13 and treated with the IP (2 µL/cm2) for 5 days. An untreated control group, a treatment control group, and a pollution control group were included. The pollution mixture included diesel particles (PM 2.5 and PM 10, 0.01%, standard reference material 1650b), benzene (1 μL/mL, Fluka, Ref. 12550), benzo[a]pyrene (1 mg/mL, Sigma, Ref. SLBS0038V), and heavy metals (Solution ICP multi-element standard V Certi Pur®, Merck; Ref.: 1.10714.0500).
Tissue histology
Tissue preparation
Post-irradiation or pollution exposure samples were fixed in buffered formalin, embedded in paraffin, cut into 5 µm sections, and mounted on Superfrost glass slides.
Sunburn cells (SBCs)
RHE was stained with hematoxylin and eosin. SBCs were identified by their dark-colored condensed and/or fragmented nucleus and eosinophilic cytoplasm, and reported as number of SBC/mm2 epidermis.
Cyclobutane pyrimidine dimer (CPDs)
CPDs were identified in RHE by in situ fluorescent immunolabeling with anti-CPD antibody (2B Scientific, Mouse monoclonal antibody IgG2ak anti-CPDs [TDM-2]) and a secondary fluorescent antibody (GAM-Alexa 488, Invitrogen, A11001) and cell nuclei were stained with propidium iodide (Sigma-Aldrich, P4170). CPD-positive cells were counted manually and compared to the total number of nuclei, reported as a percentage of the total cells. For CPDs and SBCs, each condition was performed in triplicate, and for each replicate, 5 sections were assessed, giving a total of 15 sections per condition.
Viability
Viability of skin explants was determined using Masson’s trichrome stain (Goldner variant).
Nrf2
For Nrf2 immunostaining of skin explants, a monoclonal anti-Nrf2 antibody diluted at 1:200 was used with a Vectastain Kit Vector amplifier system avidin/biotin (Vector laboratories, Burlingame, CA, USA). Three histological fields were assessed per explant, giving a total of nine assessed fields per condition.
Malondialdehyde (MDA)
MDA levels in culture medium were determined with an enhanced TBARS (thiobarbituric acid reactive substances) method. Briefly, MDA was extracted using a liquid/liquid extraction with butanol, then measured by spectrofluorimetry (excitation, 515 nm; emission, 550 nm) using an Infinite M200 Pro microplate reader (Tecan Trading AG, Männedorf, Switzerland). Levels were analyzed from four culture media for each test condition.
Gene expression analysis
RHE
Total RNA was extracted from two RHEs per condition at 4 and 24 hrs post-irradiation using TriPure (Sigma-Aldrich, USA) and subsequently pooled. RNA quantity and quality were evaluated on an Agilent Bioanalyzer 2100 (Agilent Technologies Inc., Santa Clara, CA, USA). cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche Molecular Systems Inc, Pleasanton, CA, USA). Real-time PCR was performed using the LightCycler 480 SYBR Green I Master (Roche Molecular Systems Inc, Pleasanton, CA, USA) on the following genes (primer sequences in Table 1): IL8, IL1A, MMP1, DCN, GPX3, HMOX1, PTGS2, MT1G, DEFB4A, and VEGFA. Relative gene expression was calculated with the formula (1/2number of cycles)×106 normalized to the two reference genes (RPS28 and GAPDH). A fold-change of ≥2 was considered upregulated, and ≤0.5, repressed.
 |
Table 1 Primer sequences, ultraviolet study on reconstructed human epidermis |
Explants
Total RNA was extracted using the ReliaPrep RNA tissue miniprep system (Promega Corporation, Madison, WI, USA). RNA quantity and quality were determined using BioDrop (Biodrop, Cambridge, UK) and the Experion Automated Electrophoresis System (BioRad, Irvine, CA, USA), respectively. cDNA was synthesized from 100 ng of total RNA using the iScript kit (Biorad). Real-time PCR was performed using a SYBR Green-based PrimePCR Assay (BioRad) for nine genes known to be involved in redox homeostasis (CYP1A1, GPX3, KEAP1, NRF2, and SOD2), and melanogenesis (MITF, PMEL, POMC, TYR). Relative gene expression was determined using the 2−ΔΔCq method and normalized to the reference genes GAPDH or B2M. A fold-change of ≥1.45 was considered upregulated, and a fold-change of ≤0.6, repressed.
Ethical consideration for clinical studies
Due to the cosmetic rather than medicinal nature of the IP, ethics committee approval was not required. However, in the case of the study on clinical anti-aging effects, the protocol was reviewed by the Internal Review Board (opinion no. 3129/2018) of the contract research organization that conducted the trial. Both clinical studies were performed in line with the declaration of Helsinki (1964) and its subsequent amendments and following COLIPA guidelines13 for the Evaluation of the Efficacy of Cosmetic Products, and Good Clinical Practice was maintained throughout the studies. All subjects provided signed informed consent prior to their participation.
Particle adhesion
An open-label clinical study conducted in Aix-en-Provence, France, in February 2018, assessed the effect of a single application of the IP on carbon particle adhesion in 20 healthy adult women.14 No cosmetic products were applied 24 hrs prior to the study. A 16 cm2 square was marked on both volar forearms. The IP was applied at 2 mg/cm2 by a technician to one arm, according to prior randomization; the contralateral untreated arm served as a control. The product was left to dry for 15 mins, then 4 mg of carbon particles were applied to both arms. The test areas were cleaned with 30 mL water and 75 µL neutral liquid soap and blotted dry.
Photographs were taken before product application (T0), immediately after powder application (T1) and immediately after rinsing (T2), using a C-Cube camera (Clinical Research Edition, Pixience SAS, Toulouse, France). A region of interest was marked on each photograph and, via adaptive thresholding, the area of skin with carbon particle adhesion was calculated (Kallisté software, Microvision, Evry Cedex, France).
The study endpoint was the area of skin with persistent carbon particle adhesion after washing. The effective coefficient, that is, the area of particles successfully removed after washing, relative to the amount added at the powder application stage, was calculated according to the formula ((T2−T0)−(T1−T0))/(T1−T0).
Data were assessed for normality of distribution with Shapiro–Wilk test (1% threshold). Wilcoxon test was used to assess differences between treated and control areas. Statistical significance was set at P<0.05.
Clinical anti-aging effects: complexion, firmness and elasticity, and tolerability
In July 2018, in Lisbon, Portugal, a single-center clinical study in 31 healthy adult women (mean age 56.5 years; phototypes I–IV) with signs of skin aging and atopic background was performed over 2 months to assess the product’s efficacy under normal conditions of use. The study excluded subjects with cutaneous marks, conditions (eg, pregnancy), habits, or on medications that could interfere with dermatological assessment.
The endpoints were objective change on instrumental measurement in skin complexion, elasticity and firmness, tolerability, and subject perception of efficacy and cosmetic qualities. Endpoints were assessed at baseline (D0), D28, and D56. Subjects applied the IP at home to the face and neck once daily in the morning, after washing. Subjects were instructed to use as much as they felt necessary and massage until completely absorbed, and the mean quantity used was calculated by weighing the samples at the beginning and end of the study. No other anti-aging or sun protection products or cosmetic treatments were allowed during the study; other regular hygiene habits were continued.
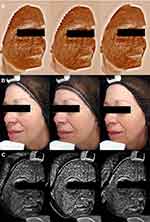
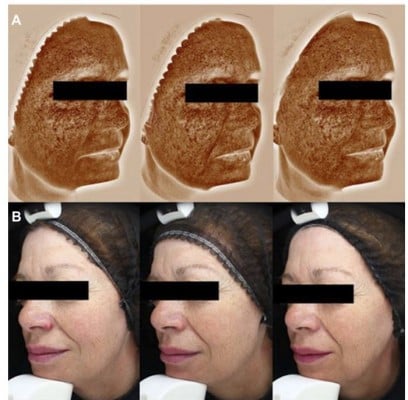
Complexion was assessed using Visia-CA (complexion analysis [Canfield, Parsippany, NJ, USA]), to quantitatively assess brown spots, visible spots, and UV spots on the face (Figure 1 shows typical images illustrative of the method).
Skin firmness and elasticity was measured in the malar area using Cutometer (Dual Cutometer MPA 580, Courage and Khazaka Electronic GmbH, Cologne, Germany) (Figure 2).
All instrumental evaluations were performed after acclimatization for at least 15 mins in a controlled room (temperature 21°C±2°C, relative humidity 55%±10%) and at a similar time of day for each subject (morning or afternoon slot).
Tolerability
Subjects recorded any reaction or discomfort on a daily observation sheet. At D0 and D56, subjects’ skin was examined for signs of irritation by a dermatologist or technician under their control, and subjects were questioned about any symptoms.
Subject opinion
Subjects completed a questionnaire on the product’s efficacy (D28 and D56) and cosmetic qualities (D56). For each item, answers were recorded on a 4-point grading scale (definitely/probably/probably not/definitely not) and results were expressed as percentage of subjects in agreement.
For statistical analysis, instrumental measurements, expressed as continuous data, were analyzed with Student t-test for normally distributed data or Wilcoxon signed rank test for non-normally distributed data. Normality was assessed with Kolmogorov–Smirnov test. All analyses were performed with SPSS 23 (IBM), and P-values <0.05 were considered significant.
Results
Protection against UV-induced damage
The product provided a high level of protection against UV-induced SBC formation, with the irradiated product-treated epidermis showing similar numbers of SBCs (mean [SEM] 9 [4.3] SBC/mm2) to the non-irradiated epidermis (7 [0.3] SBC/mm2); P<0.01 vs untreated irradiated control (191 [21.4] SBC/mm2) (Figure 3).
Following irradiation, in untreated RHE, CPDs were detected in a mean (SEM) 54% (1.2%) of cells, while in epidermis that had been treated with the IP, the cells were fully protected against CPD formation (CPDs present in 2% [0.7%]; P<0.01 for treated vs untreated irradiated samples) (Figure 4). In IP-treated epidermis, IL8, IL1A, and PTGS2 expression was lower than in untreated epidermis at 4 hrs post-UV exposure; at 24 hrs, HMOX1 and DEFB4 expression was lower in treated than in untreated RHE. Expression of DCN was also restored at this time point (Figure 5).
Protection against pollution
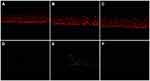
Human skin explants remained viable throughout the study. At day 5, the skin explants exposed to pollution but not treated with the IP showed intense staining for Nrf2 (described by the histologist as clear), while explants exposed to pollution but treated with the IP showed less intense Nrf2-specific staining (described as quite clear), similar to untreated unpolluted controls (Figure 6).
 |
Figure 6 Nrf2 immunostaining slides. Staining is weaker in skin treated with the product than in untreated skin. Pictures digitalized with numeric DP72 Olympus camera with CellD storing software. |
MDA levels in the untreated unpolluted group were mean (±SD) 108.5 (±16.9) nmol/L and increased to 149.1 (±9.2) nmol/L for explants exposed to pollution (P<0.05). In the sample exposed to pollution and treated with the IP, MDA levels were 114.4 (±3.2) nmol/L. This represents a 23% difference in MDA levels between the product-treated and untreated samples that were exposed to pollution (P<0.01; Figure 7).
 |
Figure 7 Mean (SD) MDA levels at day 5. |
With respect to control untreated tissues, skin exposed to pollution showed increased expression of CYP1A1 and TYR. However, in IP-treated skin this increase was not observed (Figure 8).
 |
Figure 8 Gene expression levels in pollution study. Levels expressed relative to unpolluted untreated control group. Fold-changes of <0.6 or >1.45 (yellow dashed line) were considered significant. |
Particle adhesion
The mean age of participants was 44 (27–69) years. At the end of the study, the mean (±SD) area of particle adhesion was significantly smaller in skin treated with the IP (4.96 (±4.86) mm2) than in the untreated control area (14.32 (±6.24) mm2). This corresponded to removal of 92.9% of adhered carbon particles in skin treated with the IP, vs 77.7% in untreated skin. The difference between the two arms (treated vs untreated) was statistically significant (P<0.01).
Clinical anti-aging effects: complexion, firmness and elasticity, and tolerability
There were no withdrawals or exclusions. Subject characteristics are shown in Table 2. The mean (±SD) quantity of product used per day was 0.75 (±0.38) g. No adverse reactions or discomfort were reported.
 |
Table 2 Clinical anti-aging study: subject characteristics |
On Visia complexion analysis, brown spots decreased significantly by D28, and this reduction was maintained at D56, from a mean count of 312.5 (±36.6) at baseline to 302.7 (±38.2) at D56 (−3.1%; P<0.01), and an area of 63.8 (±7.3) to 61.4 (±7.2) at D56 (−3.6%; P<0.01 vs baseline).
Visible spot count significantly decreased from 126.6 (±28.0) at baseline to 118.5 (±25.8) at D28 (P<0.01), but by D56 the change was not significant (124.3 [±29.4]; P=0.09). However, the visible spot area decreased from 39.7 (±7.4) at baseline to 37.7 (±7.8) at D56 (−5.0%; P<0.01).
UV spots decreased significantly from D28 onwards: at D56, count had decreased from 270.6 (±39.9) at baseline to 250.3 (±40.4) (−6.9%; P<0.01). Area decreased from 36.2 (±11.2) to 32.6 (±12.8) at D56 (−9.3%; P=0.02).
Firmness increased, as demonstrated by a decrease in the parameter R0, from D28 onwards, from 0.340 (±0.064) to 0.287 (±0.063) at the end of the study (−14.1%; P<0.01). Elasticity (R2) increased slightly, from 0.565 (±0.073) to 0.586 (±0.075) at the end of the study, a relative increase of 4.9%, but did not reach statistical significance (P=0.16).
The results of the subject questionnaire are shown in Table 3. Of note, 80% agreed that skin tone was more even, 74% thought skin was firmer, and 80% would recommend the product to others.
 |
Table 3 Subject questionnaire responses |
Discussion
A set of in vitro, ex vivo, and clinical studies were conducted to support the claim that an all-in-one SPF50 facial fluid containing photolyase, active biopeptides, antioxidants, and hyaluronic acid designed for urban users with busy lifestyles could provide multifaceted protection against UV radiation and pollution.
UV protection was demonstrated in an in vitro model where UV-induced SBC and CPD formation was almost totally prevented by application of the IP, with the irradiated product-treated skin having levels close to those of the non-irradiated skin. Expression of pro-inflammatory markers (IL8, IL1A, and PTGS2, at 4 hrs and DEFB4A at 24 hrs) was also lower in IP-treated dermis, suggesting it protected against UV-induced skin inflammation.
The interpretation of these results must take into account not only the high UV protection index provided by the UV filters but also the component actives of the product, in particular the DNA repair enzyme photolyase and the bioengineered pentapeptide-34 trifluoroacetate. Previous studies have shown photolyase to be effective in repairing cellular DNA damage. A 2012 clinical study15 found that topical sunscreen plus photolyase was superior to sunscreen alone in reducing CPDs, and a 2014 study16 found that a single product with SPF plus DNA repair enzymes plus antioxidants provided a greater reduction in CPDs than the sum of the effects of the individual components. In the present in vitro study, we also found the IP to be highly effective against UV-induced CPD formation, with a 100% reduction in CPD formation vs irradiated untreated control, providing support for the IP’s capacity to prevent and repair UV damage.
The second component, pentapeptide-34 trifluoroacetate, is a bioengineered peptide. When applied topically, this peptide boosts the endogenous synthesis of coenzyme Q10 (CoQ10) by upregulating PDSS1 (prenyl diphosphate synthase subunit 1), an enzyme involved in its synthesis (manufacturer data on file). CoQ10 has a potent antioxidant action17 and may therefore reduce UV-induced oxidative stress that can lead to cell apoptosis and visible signs of photoaging. Notably, treatment with the IP reduced SBC formation, lipid peroxidation – as indicated by reduced MDA levels – and the expression of antioxidative enzymes (eg GPX3, HMOX1): the antioxidant effect of CoQ10 is one likely explanation for this.
Our data also suggest that the IP can limit the damaging effects of the particulate matter component of pollution on the skin. Even though the scope of this study did not include other components of environmental pollution such as noxious gases, increasing evidence suggests that pollutants such as particulate matter exert a harmful effect on the skin by increasing oxidative stress,18 which can lead to inflammatory or allergic skin disease and skin aging.18,19 Following treatment with our all-in-one IP, levels of MDA and Nrf2, a key regulator of the antioxidative stress response,20 and expression of the CYP1A1 gene, encoding an enzyme known to play a role in the metabolic activation of polycyclic aromatic hydrocarbons21 were lower than those of untreated skin. In vitro data (data on file) suggest that 1% of pentapeptide-34 trifluoroacetate may help limit lipid peroxidation mediated by cumene hydroperoxide, an organic air pollutant.22 Our findings provide evidence of the limitation of pollution-induced oxidative effects.
While the findings of the UV and pollution preclinical studies are highly encouraging, they must be interpreted within the context of the study settings. In the pollution study, the explants used came from a single donor; a larger donor sample size would give more robust results. Regarding the insults to the skin and their resemblance to real life, the components of the pollution mixture were used to mimic the composition of pollution in a city. Nonetheless, the studies remain laboratory studies, and it would be interesting to confirm these protective effects in outdoor conditions of solar radiation and air pollution, though such studies have their own inherent limitations in methodology and controlling of numerous external factors.
Our clinical study on carbon particle adhesion demonstrated that prior application of the IP reduced residual adhesion, suggesting that the IP limits prolonged contact between pollutants and skin, potentially decreasing this inflammatory stimulus. Finally, our second clinical study found that the product was well tolerated and beneficial against signs of skin aging. Improvement of skin texture and appearance following daily application of the IP was demonstrated with a statistically significant increase in firmness (+14.1%) and a decrease in brown spots and UV spots. Such pigmentary changes may be explained by the inhibitory effect of the IP on expression of TYR and PMEL genes, which encode proteins that regulate melanin biosynthesis and melanosome formation.
The importance of user opinion and cosmetic qualities of a sunscreen product cannot be overstated. To optimize adherence, treatment regimens should be kept simple: the “best” sunscreen products are those that users will actually want to use.23 Most sunscreen users greatly value aspects such as how well the product rubs in, how it feels on the skin, and how it combines with makeup.24 The section of our study that assessed user opinion demonstrated that this product performed well on this front, as participants reported that it left skin silky and allowed it to breathe (90%), had a pleasant texture (97%), absorbed quickly (100%), had a superior texture to previous sunscreen products (84%), and that they would recommend the product (80%).
Conclusions
In skin damage and aging, the exposome theory expounds that several environmental factors act together to accelerate intrinsic skin aging.10 This theory opens the door to a novel approach in the management of skin aging, through the development of an all-in-one product that provides multifaceted protection and repair against skin aging. Such new multifunctional products must have a high cosmetic acceptability to improve user adherence.
This water-based anti-aging SPF50 sun protection formula containing photolyase, bioactive peptides and antioxidants protected and repaired against UV-induced and particulate matter component of pollution-induced skin damage and signs of aging, as determined by multiple objective measurements, and fared well in user-assessment. It represents an effective, practical option for an all-in-one daily-use product. To our knowledge, the present paper is unique in that it reports on multiple outcomes from one combination product: protection against UV radiation and pollution and clinical anti-aging effects.
Acknowledgments
A medical writer assisted in drafting the manuscript. ISDIN, the manufacturer of the product, funded the studies.
Disclosure
All authors are employees of ISDIN SA, who sponsored the study. The authors report no other conflicts of interest in this work.
References
1. D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi:10.3390/ijms140612222
2. Mancuso J, Maruthi R, Wang S, Lim H. Sunscreens: an update. Am J Clin Dermatol. 2017;18(5):643–650. doi:10.1007/s40257-017-0290-0
3. Biniek K, Levi K, Dauskardt RH. Solar UV radiation reduces the barrier function of human skin. Proc Natl Acad Sci U S A. 2012;109(42):17111–17116. doi:10.1073/pnas.1206851109
4. Liebel F, Kaur S, Ruvolo E, Kollias N, Southall M. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132(7):1901–1907. doi:10.1038/jid.2011.476
5. Soeur J, Belaïdi J, Chollet C, et al. Photo-pollution stress in skin: traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J Dermatol Sci. 2017;86(2):162–169. doi:10.1016/j.jdermsci.2017.01.007
6. Park S, Byun E, Lee J, Kim S, Kim H. Air pollution, autophagy, and skin aging: impact of particulate matter (PM10) on human dermal fibroblasts. Int J Mol Sci. 2018;19(9):2727. doi:10.3390/ijms19092727
7. Hu R, Xie X, Xu S, et al. PM2.5 exposure elicits oxidative stress responses and mitochondrial apoptosis pathway activation in HaCaT keratinocytes. Chin Med J (Engl). 2017;130(18):2205–2214. doi:10.4103/0366-6999.212942
8. Hüls A, Vierkötter A, Gao W, et al. Traffic-related air pollution contributes to development of facial lentigines: further epidemiological evidence from Caucasians and Asians. J Invest Dermatol. 2016;136(5):1053–1056. doi:10.1016/j.jid.2015.12.045
9. Addor FAS. Beyond photoaging: additional factors involved in the process of skin aging. Clin Cosmet Investig Dermatol. 2018;20(11):437–443. doi:10.2147/CCID.S177448
10. Krutmann J, Bouloc A, Sore G, Bernard B, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi:10.1016/j.jdermsci.2016.09.015
11. Vierkötter A, Schikowski T, Ranft U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130(12):2719–2726. doi:10.1038/jid.2010.204
12. Poumay Y, Dupont F, Marcoux S, Leclercq-Smekens M, Hérin M, Coquette A. A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res. 2004;296(5):203–211. doi:10.1007/s00403-004-0507-y
13. Narda M, Bauza G, Valderas P, Granger C. Protective effects of a novel facial cream against environmental pollution: in vivo and in vitro assessment. Clin Cosmet Investig Dermatol. 2018;12(11):571–578. doi:10.2147/CCID.S180575
14. Colipa, The European Cosmetics Association. Guidelines for the evaluation of the efficacy of cosmetic products; 2008. Available from: https://www.cosmeticseurope.eu/files/4214/6407/6830/Guidelines_for_the_Evaluation_of_the_Efficacy_of_Cosmetic_Products_-_2008.pdf.
15. Berardesca E, Bertona M, Altabas K, Altabas V, Emanuele E. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: clues to skin cancer prevention. Mol Med Rep. 2012;5(2):570–574. doi:10.3892/mmr.2011.673
16. Emanuele E, Spencer J, Braun M. An experimental double-blind irradiation study of a novel topical product (TPF 50) compared to other topical products with DNA repair enzymes, antioxidants, and growth factors with sunscreens: implications for preventing skin aging and cancer. J Drugs Dermatol. 2014;13(3):309–314.
17. Garrido-Maraver J, Cordero MD, Oropesa-Avila M, et al. Clinical applications of coenzyme Q10. Front Biosci (Landmark Ed). 2014;19:619–633.
18. Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol. 2017;83(4):415–423. doi:10.4103/0378-6323.199579
19. Drakaki E, Dessinioti C, Antoniou C. Air pollution and the skin. Front Environ Sci. 2014;2:11. doi:10.3389/fenvs.2014.00011
20. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi:10.1074/jbc.R900010200
21. Ma Q, Lu AY. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35:1009–1016. doi:10.1124/dmd.107.015826
22. Catalá M, Gasulla F, Pradas Del Real AE, García-Breijo F, Reig-Armiñana J, Barreno E. The organic air pollutant cumene hydroperoxide interferes with NO antioxidant role in rehydrating lichen. Environ Pollut. 2013;179:277–284. doi:10.1016/j.envpol.2013.04.015
23. Wang S, Virmani P, Lim H. Consumer acceptability and compliance: the next frontier in sunscreen innovation. Photodermatol Photoimmunol Photomed. 2016;32(1):55–56. doi:10.1111/phpp.12211
24. Xu S, Kwa M, Agarwal A, Rademaker A, Kundu R. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152(8):920–927. doi:10.1001/jamadermatol.2016.2344
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.