Back to Journals » Clinical Ophthalmology » Volume 12
A novel system, TearCare®, for the treatment of the signs and symptoms of dry eye disease
Authors Badawi D
Received 20 December 2017
Accepted for publication 2 March 2018
Published 10 April 2018 Volume 2018:12 Pages 683—694
DOI https://doi.org/10.2147/OPTH.S160403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
David Badawi
Clinical Trials Section, Central Eye Care, Arlington Heights, IL, USA
Purpose: The objective of this study was to evaluate the safety and effectiveness of the TearCare® System in adult patients with clinically significant dry eye disease (DED).
Patients and methods: This was a prospective, single-center, randomized, parallel-group, clinical trial. Subjects with DED were randomized to either a single TearCare treatment conducted at the clinic or 4 weeks of daily warm compress (WC) therapy. The TearCare procedure consisted of 12 minutes of thermal eyelid treatment immediately followed by manual expression of the meibomian glands. WC therapy consisted of once daily application of the compresses to the eyelids for 5 minutes. Subjects were followed until 6 months post-treatment. The primary effectiveness end point was defined as change from baseline to 4 weeks for tear breakup time (TBUT). Secondary effectiveness end points included meibomian gland assessment, corneal and conjunctival staining scores, and assessment of dry eye symptoms using validated questionnaires. Safety was evaluated by collecting device-related adverse events, intraocular pressure, and best spectacle-corrected Snellen Visual acuity.
Results: Twenty-four subjects were enrolled and all subjects completed 6 months follow-up. At the 1-month follow-up, TearCare subjects demonstrated an improvement from baseline in mean (±SD) TBUT of 11.7±2.6 seconds compared with an average worsening of -0.3±1.1 seconds for subjects in the WC group (p<0.0001). Significantly greater improvements in the change from baseline in meibomian gland scores, as well as corneal and conjunctival staining scores, were observed in the TearCare group. Subjects in the TearCare group also showed significantly greater improvement in dry eye symptoms as measured by the 3 questionnaires. Both treatments were well-tolerated.
Conclusion: The findings of this pilot study suggest that the TearCare System is an effective treatment option for patients with DED, with the effects on the signs and symptoms of DED persisting for at least 6 months.
Keywords: dry eye disease, meibomian gland dysfunction, iLid device, warm compresses
Introduction
Dry eye disease (DED) is among the most frequently encountered diagnoses in ophthalmology.1 In fact, it has been estimated to affect from 5% to 50% of people worldwide, with prevalence increasing with age.2 In the US, in particular, up to 40 million people suffer from or are predisposed to DED.3 Several risk factors have been associated with the development of DED. These include female gender, autoimmune disease, age, hormonal dysfunction, and contact lens wear.2
In mild cases, DED can affect visual acuity and, subsequently, routine daily tasks such as reading, driving, and using a computer. Additionally, it can result in contact lens intolerance. In more severe cases, DED can cause significant ocular surface damage and directly reduce a patient’s quality of life.2,4 Additionally, there is increasing focus on optimizing the ocular surface in patients undergoing refractive surgery, cataract surgery with premium intraocular lenses, and femtosecond laser-assisted cataract surgery. Often, DED needs to be optimally addressed in this subset of patients prior to elective surgery.5
Since 30 years ago, when DED became formally defined as an ocular disease, there has been a great shift in the understanding of the etiology of dry eye. The prevalent thinking through the 1990s was that DED resulted from aqueous deficiency.6 However, through evidence-based approaches led primarily through the International Dry Eye Workshop,6 and the International Workshop on Meibomian Gland Dysfunction (MGD),7 increasing attention has been turned to the role of the meibomian glands in DED. The current understanding suggests that patients may be diagnosed with either a predominant form of evaporative or aqueous-deficient DED, but patients frequently present with an overlapping spectrum of both etiologies, with MGD being a leading contributor to evaporative dry eye.1 Among their many functions, the meibomian glands secrete lipid onto the tear film, which retards tear film evaporation.8 The precise role of the lipid component of the tear film is the subject of intense debate, with increasing evidence that the tear film lipids provide viscoelastic properties and stabilize the tear film.9 The natural blinking mechanism has been shown to augment the normal, physiologic release of meibum from the meibomian glands.8 In 2011, Nichols et al diagrammatically demonstrated the impact of MGD on alteration of the tear film.7 It has been shown that MGD can lead to decreased tear breakup time (TBUT) and increased rate of tear evaporation which, in turn, lead to dry eye.7 In 2012, Lemp et al reported that the number of dry eye patients demonstrating MGD-associated evaporative dry eye far outnumbered those with purely aqueous-deficient DED.10
One of the key properties of the meibum that is altered in patients with MGD is an increase in the phase transition temperatures, which presumably worsens obstruction of the meibomian glands.11 It has been shown that most patients with MGD have some degree of gland obstruction. Also, increasing evidence points to the importance of clearing these obstructions during the treatment of MGD.12,13
Since most cases of MGD involve obstruction of the glands or their orifices,14 attention has been turned toward clearing these obstructions often through application of heat, mechanical force, or a combination thereof.15–17 Therapeutic levels of heat applied to meibum can melt the hardened oil and help clear the obstruction. It has been reported that meibum melts at temperatures between 32°C and 45°C (89.6°F–113°F), and severely obstructed glands may require temperatures on the higher side of that range maintained for longer periods of time for effective therapy.18–20 Because the meibomian glands are located on the inner eyelid, it is important to ensure that the inner eyelid achieves a therapeutic temperature and maintains that temperature long enough to melt meibum and clear inspissated glands.
Blackie et al conducted a series of warm compress (WC) experiments and demonstrated that a stringently controlled, externally applied WC regimen on the outer eyelid is able to achieve and maintain the effective inner eyelid temperatures required to melt meibum.20 With their controlled, labor-intensive approach using externally applied WCs maintained at 45°C by replacing the compress every 2 minutes, they were able to consistently achieve a therapeutic inner eyelid temperature that was within 2°C of the elevated outer eyelid temperature. Additionally, Olson et al15 conducted a similar series of controlled, labor-intensive WC experiments where elevated eyelid temperatures were achieved and maintained by successively replacing WCs every 2 minutes and they found an increase in both the tear film lipid layer thickness (TFLLT) and TBUT, 2 of the key measures of evaporative dry eye or MGD. In summary, it has been demonstrated that external eyelid heat therapy that can maintain an elevated temperature level for a sufficient period of time can effectively melt meibum in obstructed glands and thereby increase TFLLT and TBUT.
WC therapy for the treatment of MGD is unfortunately plagued by many shortcomings.21,22 As described above, other than a clinical study setting where an eye care provider is actively replacing WCs throughout the treatment, it is difficult to achieve and maintain a therapeutically warm (>40°C) compress.21 Often, the WC may not be sufficiently warm, or it may cool too quickly. There are concerns of overheating the compress and causing ocular injury. Additionally, a single WC may have varied temperature across its surface, often ranging >10°C (internal data). WCs also lack an ergonomic fit and are not custom-designed to conform to the tarsal plate of the eyelid for maximum efficacy. Since WC therapy is performed with the eyes closed, it does not allow for the natural blink-induced expulsion of meibum from the meibomian glands. Finally, WCs are time-consuming, labor-intensive, and require daily treatments on a lifelong basis, which create well-known patient compliance problems.
Other approaches to relieve obstructions of the meibomian glands include patient-administered eyelid massage, and expression performed by the physician,23 using manual techniques or treatment with a device such as LipiFlow (TearScience, a Johnson & Johnson Vision company, Morrisville, NC, USA).24 Patient-administered eyelid massage has many drawbacks – therapeutic consistency and efficacy cannot be ensured, and long-term compliance is suboptimal. There is also the potential for disruption of the eyelid anatomy. Additionally, the risks associated with patient-administered interventions and the development of keratoconus, retinal detachment, or other ocular conditions are unclear.25,26 In contrast, physician-facilitated manual “cold” expression of the meibomian glands has been reported to be uncomfortable and painful for the patient. Manual expression in the absence of an effective, heat pre-treatment is also limited in its ability to evacuate hardened meibum from the meibomian glands.23
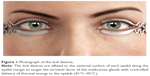
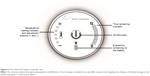
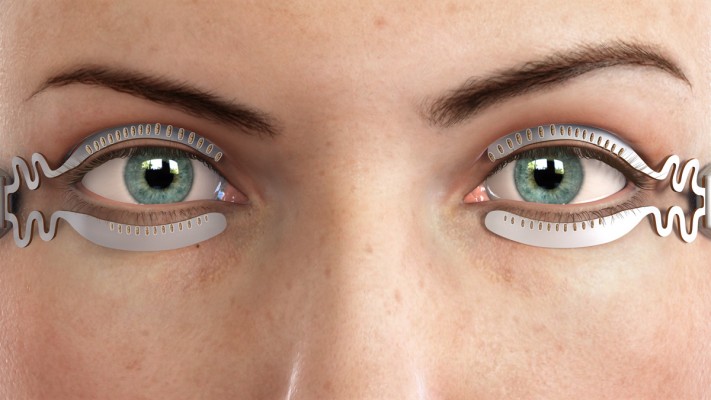
The TearCare® System is an in-office treatment for patients suffering from DED. The single-use treatment kit comprises 4 electrothermal iLid™ devices that are adhesively affixed to the tarsal plate of each eyelid. The iLid devices are shaped to conform to the eyelid anatomy to allow patients to blink during the treatment to take advantage of the body’s natural mechanism of expressing meibum during blinking (Figure 1). The iLid devices are connected via a cable to the TearCare controller (Figure 2), to deliver regulated, targeted, thermal energy across the eyelids at a consistent therapeutic temperature (41°C–45°C), which has been demonstrated to melt meibum.18–20 The thermal cycle is followed immediately by in-office, manual meibomian gland expression with the expression forceps to help ensure a thorough evacuation of any residual meibomian gland obstructions not cleared during the thermal cycle. This allows for a precise and controlled delivery of thermal energy to the eyelids and 2 levels of meibomian gland obstruction expression – natural blinking expression and mechanical expression performed on the patient by the eye care provider.
What is described here is a prospective, randomized, parallel-group pilot study comparing the safety and efficacy of standardized, at-home, daily WC therapy, the standard of care, to a single in-office TearCare treatment.
Patients and methods
This pilot study was conducted as a prospective, single-center, randomized clinical trial in full accordance with the tenets of the Declaration of Helsinki and US Food and Drug Administration regulations for the protection of human subjects in medical research. All assessments were conducted at the clinical site by a board-certified ophthalmologist and subjects completed the self-administered questionnaires at the clinical site, as well. Study-related documents and procedures were reviewed and approved by the Aspire Institutional Review Board (Aspire IRB, Santee, CA, USA) as a non-significant risk device study. The study was registered at ClinicalTrials.gov; the registry number is NCT03006978.
All subjects who participated in the study were required to read and sign the IRB-approved informed consent form prior to the initiation of any study-related procedures. Participants in the study were screened from the patient population at the clinical site. Patients were screened for suitability for inclusion in the study based on the following criteria: at least 18 years of age, reports of dry eye symptoms within 3 months of the screening assessed through the Standard Patient Evaluation for Eye Dryness II (SPEED II)27,28 questionnaire score of ≥6 and a Schirmer I tear test score of ≤10 mm in at least 1 eye and/or a TBUT of <10 seconds in at least 1 eye.
Patients were excluded from the study based on the presence or history of ocular inflammation, infection, abnormalities and/or ocular or systemic conditions that may have prevented the patient from completing the study or could have confounded the results of the study assessments in the opinion of the Investigator. Patients were also excluded from the study if they had a history of ocular surgery, had a recent history (past 30 days) of topical ophthalmic medication use, including antibiotics, steroids, non-steroidal anti-inflammatory drugs, or required the chronic use of topical ophthalmic medications. Patients who were currently using prescription medications related to treatment of their DED were required to go through a washout period (2 months for anti-inflammatory medications, including cyclosporine or lifitegrast, and 2 weeks for tetracycline class agents such as doxycycline or minocycline) between the screening and baseline visits to be eligible for study participation. Patients were allowed to continue using artificial tears if needed.
A total of 6 or 7 study visits (subjects who did not require a medication washout period could combine the screening and baseline visits) were required for completion of the study: screening, baseline (day 0), day 1, weeks 2 and 4, 3 and 6 months. The screening visit was used to determine the subject’s eligibility to enroll in the study based on the inclusion/exclusion criteria. The subject’s demographic, medical history, medication use (ocular and systemic), and ocular history were all obtained at the initial screening/baseline visit. The subject’s best-corrected visual acuity (BCVA) was evaluated at each study visit. A slit-lamp examination was also conducted at each study visit; corneal and conjunctival staining was evaluated, along with TBUT and a meibomian gland assessment. The SPEED, and ocular surface disease index (OSDI)29 questionnaires were administered at the screening/baseline, weeks 2 and 4, 3- and 6-month study visits. The Symptom Assessment in Dry Eye (SANDE) questionnaire30 was administered at the screening/baseline, week 4, 3- and 6-month study visits. Subjects’ intraocular pressure (IOP) was measured at the screening/baseline, week 4, 3- and 6-month study visits. Subjects completed the questionnaires prior to the clinical evaluations. Clinical evaluations were conducted in the following order: visual acuity assessment, TBUT, corneal fluorescein staining, conjunctival lissamine green staining, meibomian gland assessment, slit-lamp examination, and IOP measurement. Both eyes were evaluated for each subject.
The slit-lamp examination included evaluation of the ocular surface and adnexa for the presence of erythema, conjunctival discharge, observation of the tear meniscus, thickening of the lid margins, and telangiectasia. Using a Korb Meibomian Gland Evaluator (TearScience), the meibomian gland assessment was conducted by evaluating the consistency of the secretions that were observed upon expression of meibomian glands in the nasal, central, and temporal regions of the lower eyelids. The central, consecutive 5 glands in each region were evaluated. The instrument was held at the eyelid margin for 10–15 seconds, and the secretions were graded on a 0–3 scale for each gland (0=no expression; 1=toothpaste; 2=cloudy; and 3=clear), with a 0–45 score range for each eye.24
The TBUT was evaluated to monitor the stability of the tear film. Fluorescein was introduced to the temporal inferior fornix using dry eye test strips (Amcon Laboratories, St Louis, MO, USA). The TBUT was evaluated by measuring the time to breakup of the tear film following a complete blink when viewed through the slit-lamp using a cobalt blue filter. The TBUT was recorded using a stopwatch for each eye as the average of 3 measurements at each visit.
Corneal (fluorescein) and conjunctival (lissamine green) staining scores were recorded to assess the integrity of the ocular surface. The severity of staining was assessed and quantified using the National Eye Institute/Industry Grading System31 scoring 5 regions of the cornea and 6 regions of the conjunctiva using a scale from 0 to 3 (normal–severe). The scores for the regions of the cornea and conjunctiva were added to obtain a total score that ranged from 0 to 15 or 0 to 18, respectively. The Schirmer test was conducted to evaluate the subject’s aqueous tear production. A Schirmer I test (without anesthesia) was performed by placing a Schirmer strip into the lower fornix. The length of the strip that was wetted was measured and recorded for each eye after 5 minutes.
The SPEED, OSDI, and SANDE questionnaires are all well-validated instruments that are widely used assessment tools for dry eye indications. The SPEED questionnaire was used to evaluate the symptoms of study subjects. The SPEED survey measures the severity (0–4 scale) and frequency (0–3 scale) of DED symptoms.27 The SPEED score was calculated by adding the total of the frequency and severity scores; a score of ≥6 may be an indicator of DED. The OSDI questionnaire is a patient-based outcome measure used to assess ocular symptoms, their impact on patient vision-related functioning, and environmental factors triggering the symptoms. Each question is graded on a 0–4 scale, and a total score (0–100) is calculated based on the subject’s responses (sum of scores ×25)/the number of questions answered.29 The overall OSDI score defines the level of ocular surface disease as normal (0–12 points), mild (13–22 points), moderate (23–32 points), or severe (33–100 points). In the study by Miller et al, a minimal clinically important difference for the OSDI ranges from 7.0 to 9.9, depending on the category of the disease prior to treatment.32
The SANDE questionnaire is a patient-based outcomes instrument that uses 2 visual analog scales (VAS) to measure the frequency (0–100 mm; never – all the time) and severity (0–100 mm; very comfortable – very severe) of subject’s sensation of ocular dryness or irritation.30,33 Since research with VAS instruments has shown that knowledge of previous ratings may assist patients in making accurate and more consistent estimates of change relative to previous experience,34,35 at each follow-up time point, subjects were provided with their prior SANDE responses.
Adverse events (AEs) were recorded at each study visit.
Study design
Once a subject’s eligibility for study participation had been confirmed and all baseline assessments had been performed, each enrolled subject was randomized in a 1:1 ratio to either the TearCare treatment group or the WC treatment group. Given the identifiable difference between treatments and that this was a single investigator study, masking was not feasible.
TearCare system
The TearCare system consists of the controller, charging nest, charging adaptor, and the single use iLid devices that are attached to the upper and lower eyelids of both eyes. The subject’s eyelids were cleaned with a make-up removal wipe prior to treatment to remove makeup. Subjects randomized to the TearCare group underwent the TearCare treatment at the baseline visit. An iLid device was affixed to the external surface of each eyelid along the eyelid margin to target the terminal ducts of the meibomian glands. Next, the controller was activated and treatment initiated. This consisted of the controlled and targeted delivery of thermal energy to the functional eyelids (range 41°C–45°C) for 12 minutes. Subjects were queried throughout to ensure comfort during treatment. Subjects were encouraged to blink normally during the procedure, thereby harnessing the eye’s natural meibum expression forces during treatment when meibum is in its melted phase. Next, the iLid devices were removed, and a drop of 0.5% tetracaine was applied to the conjunctival fornix of each eye. Meibomian gland expression was performed by the investigator under direct slit-lamp visualization using meibomian gland forceps (Rhein Medical Inc, St Petersburg, FL, USA).
Standardized daily WCs
Daily WC therapy was performed using MGDRx bags (The Eye Bag Company, Halifax, UK). The compress bag was placed in a microwave and heated for 30 seconds. Subjects who were randomized to the WC group received their first 5-minute WC treatment in the investigator’s clinic to ensure they understood the instructions for use. Subjects were provided with the WC device, and instructed in how to heat and use the device for treatments at home. Subjects were provided a log for daily use and instructed to apply the WC at home 1 time a day (5 minutes/day) for 4 weeks.
Study end points
The primary efficacy end point was the change from baseline in TBUT at 4 weeks. Secondary efficacy end points included the change from baseline in meibomian gland assessment scores, corneal and conjunctival staining scores, and the results for the SPEED, OSDI, and SANDE questionnaires. Safety end points included the recording of AEs, changes in IOP, and BCVA.
Statistical analyses
No calculations with respect to the sample size of the study population were conducted due to the exploratory nature of this pilot study. The primary efficacy population was an intent-to-treat population consisting of all randomized eyes, analyzed according to the group to which they were randomized. For each baseline variable, the mean (±SD) for the TearCare system and WC treatment groups and the difference between the treatment group mean values were calculated. Non-paired t-tests were performed to test for statistically significant differences in baseline characteristics. The pre-specified primary efficacy end point was change from baseline in TBUT at 4 weeks. Thus, for comparisons between treatment groups, p-values are the result of separate tests at each follow-up time. For eye-level measurements of TBUT, corneal staining, conjunctival staining, and meibomian gland assessment score, statistical significance was determined using simple mixed-effects linear models that allowed for correlation between eyes within subjects (via likelihood ratio χ2 tests). These simple mixed-effects models included a fixed effect for the change from baseline and a random effect (a random intercept since there was only 1 time point) per person to account for correlation between eyes within subject. In these simple analyses, each follow-up time was analyzed separately, and there were no other covariates included in the model. For the SPEED, SANDE, and OSDI scores, where there is only a single measurement per person, no adjustment for correlation is necessary. For these measurements, significance was determined using a 2-sample t-test that compared change from baseline at each follow-up time. All analysis variables passed a visual normality check using normal quantile plots. The pre-specified primary end point (a single variable and time point) requires no correction for multiplicity. No corrections for multiplicity were performed for secondary analyses. A p-value ≤0.05 was the threshold for determining statistical significance. All statistical analyses were carried out using R (version 3.3.3).
Results
Study populations
A total of 24 subjects (48 eyes) met the inclusion criteria and were subsequently enrolled to participate in the trial. Half of the subjects (12 subjects; 24 eyes) were randomized to each group. All 24 subjects returned for each follow-up visit and completed the trial. The demographic information describing the study population is summarized in Table 1. It should be noted that the 2 groups were demographically similar; the mean age ± SD was 69.3±11.5 years and 66.1±15.1 years for subjects in the TearCare group and WC group, respectively. All participants in the study were female. The TearCare treatment group received a single treatment at the conclusion of their baseline visit. The WC group completed daily logs confirming that they complied with the WC schedule of 5 minutes daily for a total of 4 weeks, with the exception that 1 subject did not record treatment on 1 day. The baseline scores for the study end points were similar between the treatment groups with the exception of the meibomian gland score, in which the mean ± SD scores for the TearCare treatment group (6.3±3.6) were worse than the mean scores for the WC group (9.0±4.3). All the subjects had a TBUT indicating an evaporative component to their DED, the mean TBUT measurements were 3.1±0.8 seconds for the TearCare group and 3.3±1.0 seconds for the WC group. Additionally, 71% of patients had a Schirmer I score in at least 1 eye that indicated some degree of aqueous deficiency contributing to their DED. These baseline characteristics are summarized in Table 2.
  | Table 1 Baseline demographics of the study population |
TBUT (primary end point)
A significant improvement in TBUT from baseline was observed in the TearCare treatment group. At 4 weeks, the mean TBUT in the TearCare treatment group had increased by nearly 12 seconds (11.7±2.6 seconds) compared to a decline in TBUT for the WC group (−0.3±1.1 seconds). The significant improvement in TBUT for the TearCare group was seen on the day 1 visit (p<0.0001) and these statistically significant differences persisted through all the 6-month visits. No significant differences in the mean change from baseline of the TBUT were observed for any of the follow-up visits of the WC group. The mean TBUT scores for each treatment group are presented in Figure 3.
Corneal and conjunctival staining
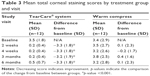
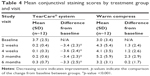
Significant improvements in corneal and conjunctival staining, indicating a reduction in ocular pathology correlated with the severity of DED, were observed for subjects in the TearCare group. At 4 weeks, corneal and conjunctival staining significantly decreased in the TearCare group from a mean score of 3.5±1.8 to 0.2±0.4 and 3.7±2.5 to 0.1±0.3, respectively (p<0.0001 for the comparison of the change from baseline between groups for both end points). There was no significant change for either corneal or conjunctival staining in the WC group at 4 weeks. There were statistically significant improvements in terms of corneal staining for the TearCare group as compared to minimal changes for the WC group over the entire 6-month follow-up period. Tables 3 and 4 summarize the mean corneal and conjunctival staining scores at each visit, respectively.
Dry eye symptoms
All subjects completed 3 different questionnaires to assess their degree of DED symptoms at the baseline and follow-up visits. The questionnaires included the SPEED, OSDI, and SANDE patient-based outcomes measures.
SPEED
Subjects in the TearCare and WC groups had similar mean SPEED scores at baseline (15.7±5.2 vs 14.4±3.8, respectively). A score of ≥6 on the SPEED II is generally considered an indication that the patient has symptoms of DED. Subjects in the TearCare group consistently showed a significantly greater improvement from baseline in average SPEED scores throughout follow-up compared to subjects in the WC group. Figure 4A graphically demonstrates the SPEED results between the 2 groups. At the primary time end point of 4 weeks, the mean SPEED score in the TearCare group had decreased from 15.7±5.2 at baseline to 7.8±3.5 compared to a decrease from 14.4±3.8 to 12.6±3.3 for the WC group (p=0.001). The treatment effect was most pronounced at 3 months; however, at all time points, the TearCare subjects demonstrated significantly more improvement in SPEED scores compared with WC subjects. Subjects in the WC group also saw some improvements in SPEED scores, peaking at 3 months when the average SPEED score improved −2.6±4.3 points.
OSDI
According to the OSDI scale, subjects in both the TearCare and the WC group had a dry eye grade of severe at baseline (mean values of 41.0±18.4 and 33.0±19.9, respectively). At 4 weeks follow-up, TearCare subjects’ mean improvement from baseline was −25.3±17.9, which represents a clinically meaningful improvement32 and which was also significantly better than the −8.4±17.1 mean improvement observed in the WC group (p=0.03). Following treatment, TearCare subjects experienced, on average, clinically and statistically significant greater improvements in their quality of life and dry eye symptoms based on their reported OSDI scores at all time points for at least 6 months, compared with subjects in the WC group (Figure 4B). Subjects in the WC group experienced some improvements in their OSDI scores at 1 month, but this improvement was not sustained up to 3 and 6 months.
SANDE
The mean VAS scores for the SANDE questionnaire were similar at baseline between groups, with subjects in the TearCare group having a worse score (higher) than subjects in the WC group (64.9±25.9 and 55.9±31.5, respectively). The mean SANDE score for subjects in the TearCare group improved from 64.9±25.9 at baseline to 40.2±18.8 at the primary end point time of 4 weeks compared with a slight worsening for the WC group, in which the score increased from 55.9±31.5 to 57.5±25.7. TearCare subjects showed a mean change (improvement) in SANDE score from baseline to 6 months of −19.0±22.7 vs a mean change (worsening) from baseline to 6 months of 6.2±29.3 in the WC group (p=0.03) (Figure 4C). At all follow-up time points, the TearCare subjects showed a significant improvement in dry eye symptoms as measured by the SANDE, whereas the WC subjects showed no significant changes over time.
Overall, both groups did demonstrate improvement in the scores for these questionnaires as shown in Figure 4; however, the improvement in symptoms as measured by these questionnaires were significantly greater in the TearCare group at all time points except for the comparison between score improvement specifically for the SANDE questionnaire at the 3-month visit.
Meibomian gland scores
The quality of the meibomian gland secretions in both subject groups was poor at the baseline visit, reflected by the mean scores of 6.3±3.6 and 9.0±4.3 for the TearCare and WC groups, respectively. At the 4-week primary end point time, the TearCare group had a statistically significant improvement (increase) in the mean meibomian gland score from a baseline score of 6.3±3.6 to 41.0±2.1 compared with a statistically insignificant change in the WC group from 9.0±4.3 to 8.2±4.0. Compared with the WC group, the TearCare group revealed statistically significant improvement in meibomian gland scores (p<0.0001), reflecting an improvement in the quality of the meibomian gland secretions, compared with the WC group (Figure 5).
Safety
The treatments were well-tolerated by subjects in both groups. Immediate post-treatment and day 1 examinations of TearCare subjects found no evidence of heat-related AEs on the eyelids or cornea surface. Furthermore, neither group of subjects reported any AEs or experienced significant changes in IOP or visual acuity throughout the 6-month study.
Discussion
The primary purpose of this study was to evaluate the safety, effectiveness, and sustainability of a single, in-office treatment with the novel TearCare System compared with daily, at-home standardized WC therapy applied over a 4-week period. Subjects were followed up to 6 months, and all subjects were compliant with their treatments and completed the study. This study was motivated, in part, by the unmet need in historic DED treatment and the recognized shortcomings in the use of at-home WCs as a long-term therapy to relieve the signs and symptoms of DED, including persistence, labor intensity, variability, poor ergonomics, heat treatment gaps, closed eye design, poor compliance, and safety.
Overall, both the TearCare and WC groups demonstrated subjective improvement in symptoms using 3 different accepted clinical patient-based outcomes measures, the SPEED, OSDI, and SANDE questionnaires. For most time points measured, the TearCare group fared better, and the results were statistically and clinically significant.
In terms of the objective measurements such as TBUT, meibomian gland scores, and corneal and conjunctival staining, the TearCare group demonstrated an immediate, statistically significant, and sustained improvement from baseline out through 6 months. No such improvement was seen in the WC group. Particularly notable were the improvements seen in the TBUT and meibomian gland scores of the TearCare group. Moreover, the improvement in patients’ signs and symptoms in the TearCare group compared with the WC group was also significant out to 6 months.
The TearCare System delivers heat (41°C–45°C) to the outer surface of the eyelid for 12 minutes. This temperature range is intended to allow effective melting of obstructed meibum,18,19,36 while at the same time posing no risk to the patient.37,38 The safety of this level of heat applied to the eyelids has been demonstrated by Blackie et al in which WCs heated to 45°C were successively applied for 30 minutes with no reported heat-related injury.20 The results of this TearCare study confirm the safety of this approach as no AEs were observed in the TearCare group. Furthermore, the significant improvement in meibomian gland scores observed in this study demonstrates that the safe and consistent application of heat directly to the tarsal plates of the functioning eyelids is therapeutic and effective in melting meibum. Abnormalities in meibomian gland secretions associated with MGD are recognized as contributors to evaporative dry eye, a common component in the clinical presentation of many patients with DED.1
This trial does have some limitations. Given the nature of the trial and that it was a single investigator study, it was not possible to effectively mask the subjects or the investigator/assessor. Masking of the investigator/assessor will be addressed in a planned, larger prospective, multicenter, randomized trial where the treatments and evaluations will be conducted by different investigators. Given the difference in the devices and treatment regimens (1-time treatment vs daily treatment), it is not possible to mask subjects. Additionally, while the results of this study were often statistically significant, a larger number of subjects enrolled at different centers, as is planned in a future study, will enhance the evidence base for this approach.
Unlike previous comparable trials that evaluated subjects for 30 days, such as the one that studied the LipiFlow System24 and showed some clinical improvement, this trial followed the 2 treatment groups out to 6 months. The results of this pilot study indicate that the application of targeted thermal energy to the outer surface of the eyelids resulted in sustained improvements in the signs and symptoms of DED. The subjective and objective findings show that a single TearCare treatment yields an effect that is sustained for at least 6 months. Further studies could evaluate whether a similar effect can be reproduced with retreatment at 6–8 months.
Conclusion
A single treatment with the TearCare system, in this clinical trial, demonstrated a sustained and statistically significant improvement in the signs and symptoms of DED at the primary end point time of 4 weeks and at each follow-up visit up to 6 months. Results from this study provide a preliminary demonstration of the safety and effectiveness of the TearCare system in treating the signs and symptoms of DED. A larger, multicenter, randomized, prospective trial is planned to further evaluate this treatment for DED.
Acknowledgments
The author would like to thank Gary Foulks, MD and Kelly Nichols, OD for providing editorial assistance on the manuscript. Gerry Gray, PhD for performing the statistical analysis of the data. Kurt Brubaker for his assistance in preparing and editing the manuscript. This study was sponsored by Sight Sciences, Inc. (Menlo Park, CA, USA). The sponsor participated in the design of the study and data collection. The sponsor also reviewed the manuscript for accuracy and assessment of the presentation of any proprietary information.
Disclosure
In addition to practicing as a board-certified ophthalmologist, David Badawi is an employee of Sight Sciences, Inc. The author reports no other conflicts of interest in this work.
References
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. | ||
Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. | ||
Ding J, Sullivan DA. Aging and dry eye disease. Exp Gerontol. 2012;47(7):483–490. | ||
Fiscella RG. Understanding dry eye disease: a managed care perspective. Am J Manag Care. 2011;17(Suppl 16):S432–S439. | ||
Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017;11:1167–1173. | ||
The definition and classification of dry eye disease: report of the definition and classification subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5(2):75–92. | ||
Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. | ||
Knop E, Knop N, Schirra F. [Meibomian glands. Part II: physiology, characteristics, distribution and function of meibomian oil]. Ophthalmologe. 2009;106(10):884–892. German. | ||
Willcox MDP, Argueso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366–403. | ||
Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. | ||
Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(6):3805–3817. | ||
Korb DR, Blackie CA. Restoration of meibomian gland functionality with novel thermodynamic treatment device–a case report. Cornea. 2010;29(8):930–933. | ||
Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res. 2011;36(2):79–87. | ||
Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29(12):1333–1345. | ||
Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29(2):96–99. | ||
Craig JP, Blades K, Patel S. Tear lipid layer structure and stability following expression of the meibomian glands. Ophthalmic Physiol Opt. 1995;15(6):569–574. | ||
Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment of meibomian gland dysfunction. Adv Exp Med Biol. 1994;350:293–298. | ||
Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–165. | ||
Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78(3):347–360. | ||
Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008;85(8):675–683. | ||
Lam AK, Lam CH. Effect of warm compress therapy from hard-boiled eggs on corneal shape. Cornea. 2007;26(2):163–167. | ||
Freedman HL, Preston KL. Heat retention in varieties of warm compresses: a comparison between warm soaks, hard-boiled eggs and the re-heater. Ophthalmic Surg. 1989;20(12):846–848. | ||
Blackie CA, Korb DR. Meibomian gland expression: forces of expression, types of secretion and the limitation of resulting pain. Invest Ophthalmol Vis Sci. 2010;51(13):3385. | ||
Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the lipiflow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404. | ||
Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye (Lond). 2014;28(2):189–195. | ||
Panikkar K, Manayath G, Rajaraman R, Saravanan V. Progressive keratoconus, retinal detachment, and intracorneal silicone oil with obsessive-compulsive eye rubbing. Oman J Ophthalmol. 2016;9(3):170–173. | ||
Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204–1210. | ||
Asiedu K, Kyei S, Mensah SN, Ocansey S, Abu LS, Kyere EA. Ocular Surface Disease Index (OSDI) Versus the Standard Patient Evaluation of Eye Dryness (SPEED): a study of a nonclinical sample. Cornea. 2016;35(2):175–180. | ||
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. | ||
Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–57. | ||
Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221–232. | ||
Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. | ||
Amparo F, Schaumberg DA, Dana R. Comparison of two questionnaires for dry eye symptom assessment: the ocular surface disease index and the symptom assessment in dry eye. Ophthalmology. 2015;122(7):1498–1503. | ||
McMonnies CW. Measurement of symptoms pre- and post-treatment of dry eye syndromes. Optom Vis Sci. 2016;93(11):1431–1437. | ||
Miller MD, Ferris DG. Measurement of subjective phenomena in primary care research: the visual analogue scale. Fam Pract Res J. 1993;13(1):15–24. | ||
Terada O, Chiba K, Senoo T, Obara Y. [Ocular surface temperature of meibomian gland dysfunction patients and the melting point of meibomian gland secretions]. Nippon Ganka Gakkai Zasshi. 2004;108(11):690–693. Japanese. | ||
Moritz AR, Henriques FC. Studies of thermal injury: the relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol. 1947;23(5):695–720. | ||
Despa F, Orgill DP, Neuwalder J, Lee RC. The relative thermal stability of tissue macromolecules and cellular structure in burn in-jury. Burns. 2005;31(5):568–577. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.