Back to Journals » International Journal of Nanomedicine » Volume 14
A novel strategy of transferring NIS protein to cells using extracellular vesicles leads to increase in iodine uptake and cytotoxicity
Authors Son SH, Gangadaran P , Ahn B
Received 4 October 2018
Accepted for publication 8 February 2019
Published 7 March 2019 Volume 2019:14 Pages 1779—1787
DOI https://doi.org/10.2147/IJN.S189738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Seung Hyun Son,1,2,* Prakash Gangadaran,1,2,* Byeong-Cheol Ahn1,2
1Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Daegu, Republic of Korea; 2Department of Nuclear Medicine, Kyungpook National University Hospital, Daegu, Republic of Korea
*These authors contributed equally to this work
Background: This study was designed to explore a novel approach for transferring NIS protein to cells using extracellular vesicle (EV) and enhancing iodine avidity in hepatocellular carcinoma (HCC) cells.
Methods: We transfected the HCC cells (Huh7) with NIS gene, designated as Huh7/NIS, and isolated the EVs from them. Presence of NIS protein in EVs and EV-mediated transport of NIS protein to recipient Huh7 cells were tested using Western blotting. We also examined radioiodine uptake in Huh7 cells treated with EV-Huh7/NIS.
Results: Successful transfer of NIS protein into Huh7 cells was confirmed by WB and microscopy. EVs showed high levels of NIS protein in them. Treatment of Huh7 cells with EV-Huh7/NIS increased the NIS protein level and enhanced 125I uptake in recipient Huh7 cells. In addition, EV-huh7/NIS pre-treatment enhanced the cytotoxicity of 131I therapy against Huh7 cells by inducing increased DNA damage/increased γH2A.X foci formation.
Conclusion: This is the first-of-its-kind demonstration of successful transportation of the NIS protein to cells via EVs, which increased radioiodine uptake. This approach can revert radioiodine-resistant cancers into radioiodine-sensitive cancers.
Keywords: sodium iodide symporter (NIS), extracellular vesicle, iodine uptake, hepato-cellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a primary tumor of the liver; one of the most common causes of cancer-associated mortality.1 Very poor prognosis of HCC is primarily because it is usually diagnosed at late and advanced stages. In addition, the best curative options are either tumor resection or liver transplantation. These are not possible therapeutic approaches for most HCC patients. NIS is a fundamental plasma membrane glycoprotein with 13 trans-membrane domains that actively transfers both sodium and iodide together through the cytosol from extracellular-fluid by facilitated diffusion and the sodium/potassium ATPase pump; the action of NIS is moved by low internal sodium concentration in thyroid epithelial cells.1,2
Although 131I is currently an effective therapeutic option for thyroid cancers that express NIS,3,4 a considerable number of metastatic thyroid carcinomas may not express adequate amounts of the protein and are classified as radioiodine-refractory thyroid cancers.3,5 Although HCC is well-known to express low levels of NIS, it can be treated by 131I therapy only by artificially introducing NIS into the cancer cells.4,6 Furthermore, HCC is extremely resistant to chemotherapy or radiotherapy or both.2,3 Therefore, a novel therapeutic strategy is needed for unresectable HCC.
Extracellular vesicles (EVs) are nano-sized vesicles secreted by almost all cells, they can deliver varieties of molecules, such as lipids, proteins, and nucleic acids.7,8 Substances contained inside EVs may be released into the extracellular milieu locally or at some distance to establish local as well as long-distance communication between two cells.9 EVs are involved in transferring active proteins to recipient cells by interacting with specific molecules on the surface of target cells and can promote downstream signaling responses of the target cells.10–13 In few cases, direct attachment of EVs with target cells resulted in either fusion of the EVs with the cells or internalization of EVs into the cells.14
In this study, we hypothesized that EV-mediated transfer of NIS protein to HCC cells might enhance the iodine uptake into these cells. Here, we have developed NIS-expressing HCC cells (Huh7 cells) and produced EVs with NIS protein from the recipient HCC cells. Then, we transferred the NIS protein, using EV technology, into parent HCC cells and studied the biological function of the transferred NIS protein in the recipient HCC cells, including iodine avidity and cytotoxicity.
Materials and methods
Ethics statement
HCC cells (Huh7) were purchased from commercial provider “Korean Cell Line Bank” (KCLB; Seoul, Republic of Korea).
Cell culture and establishment of a cell line
HCC cells (Huh7) were purchased from the Korean Cell Line Bank (KCLB; Seoul, Republic of Korea). Huh7 cells were grown in DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) comprising 10% FBS (Hyclone, Thermo Fisher Scientific) and 1% penicillin–streptomycin (Gibco, Thermo Fisher Scientific) and cultured in a 5% CO2 atmosphere at 37°C. Human NIS (hNIS) gene was obtained from Dr JK Chung (Seoul National University) and the pEGFP–NI vector was purchased from Clontech (Mountain view, CA, USA). The downstream region of cytomegalovirus (CMV) promoter included the hNIS and EGFP genes, internal ribosomal entry site (IRES) was used to allow co-expression of each gene under the control of the CMV promoter. For transfection, the Huh7 cells were seeded and cultured in a 5% CO2 atmosphere at 37°C for 24 hours. When cell confluency reached 60%–70%, the purified-recombinant plasmid (containing hNIS and EGFP) was transfected by using Lipofectamine plus reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s guidelines. The stable cell line was established as (Huh7/NIS) expressing hNIS, and EGFP using flow cytometry (FACSorter, BD Biosciences, San Jose, CA, USA).
Western blotting
Western blotting was carried out as previously described.15 Cell or EV lysates were prepared in lysate RIPA buffer (Thermo Fisher Scientific), to which a cocktail of protease inhibitor was added (Sigma-Aldrich Co., St Louis, MO, USA). An equal amount of protein was loaded and run on a 10% SDS-PAGE gel. The proteins were moved from the gel to a PVDF membrane (Merck Millipore, Billerica, MA, USA) and probed, first, with the primary, and followed by HRP-conjugated secondary antibody. The signals were detected using a chemiluminescence detection system (GE Healthcare, Chicago, IL, USA), according to the manufacturer’s protocol. Primary antibodies used: anti-mouse-NIS (Thermo Fisher Scientific; dilution: 1:2,500), anti-rabbit-CD63 (Abcam, Cambridge, UK; dilution: 1:2,000), anti-rabbit-GM130 (Abcam; dilution: 1:3,000), anti-mouse-GAPDH (Santa Cruz Biotechnology Inc., Dallas, TX, USA; dilution: 1:5,000) and anti-rabbit-β-actin (Cell Signaling Technology, Danvers, MA, USA; dilution: 1:5,000). HRP-conjugated anti-rabbit (Cell Signaling Technology; dilution: 1:5,000) and anti-mouse (Cell Signaling Technology; dilution: 1:5,000) secondary antibodies.
Immunofluorescence
Immunofluorescence assay was performed as described previously.7 Huh7/NIS cells were blocked with 3% BSA in PBS and probed with NIS antibody (Abcam; dilution: 1:50) overnight. Cells were washed with PBS (three times), then probed with secondary antibody conjugated with Alexa Fluor-488 (Abcam; dilution: 1:300). Cells were washed with PBS (three times) and cells were mounted using DAPI-containing mounting medium (Vector Laboratories, Burlingame, CA, USA). Images were attained with a Zeiss super-resolution confocal microscope (LSM 5 Exciter, Zeiss, Oberkochen, Germany).
Isolation of EVs
The Huh7 and Huh7/NIS cells were cultured as mentioned previously, and EV-depleted FBS (18 hours at 120,000× g at 4°C) was used for all EV procedures. EVs were enriched as described previously.1 Briefly, 1×106 cells were seeded into 100 mm culture dishes. Culture supernatants were collected when cells reached 80%–90% confluency. The Huh7/NIS supernatant was first centrifuged at 300× g for 10 minutes, second at 1,500× g for 15 minutes, and third at 2,500× g for 20 minutes (to remove debris and dead cells). The supernatant was passed through a 0.45 μm syringe filter. Open-Top Thinwall Ultra-Clear Tube (Beckman Coulter, Brea, CA, USA) was used as ultracentrifuge. Each tubes were filled with 35 mL of culture supernatant. Samples were centrifuged at 100,000× g for 60 minutes. Then, pellets of EVs were washed with PBS and centrifuged again at 100,000× g for 60 minutes. The pellets were reconstituted in PBS, and either used immediately or stored at −80°C. All centrifugations were carried out by using the Optima™ L-100 XP ultracentrifuge (Beckman Coulter). All centrifugations were done at 4°C. Total protein contents of EVs were measure by BCA assay kit (Thermo Fisher Scientific).
Transmission electron microscopy (TEM)
EVs from Huh7/NIS cells (EV-Huh7/NIS) were resuspended in 2% paraformaldehyde (100 μL), then 5 μL EVs were moved to the Formvar-carbon-coated EM grids (Electron Microscopy Sciences, Redding, CA, USA) and dried in air for 20 minutes. PBS (50 μL) was added on a parafilm sheet and the grids were transferred onto the PBS using sterile forceps for washing. The grids were then moved to 1% glutaraldehyde (50 μL) and left in room temperature for 5 minutes. The grids were washed in distilled water for 2 minutes. EVs in grids were negatively stained with 2% uranyl acetate followed by washing with PBS seven times, drying, and observation on HT 7700 transmission electron microscope (Hitachi Ltd., Tokyo, Japan) to image the EVs.
Electrophoretic light scattering (ELS) analysis
PBS-resuspended EV-Huh7/NIS was further diluted 200–400-fold with distilled water. Size, distribution, and Zeta potential of EVs were determined with an ELS-Z (Otsuka Electronics, Osaka, Japan). Zeta potential measurements were carried out at 25°C.
In vitro 125I uptake assay
To study 125I uptake, Huh7 cells (1.25×105) were seeded in 24-well plates for 24 hours and incubated with EV-Huh7/NIS for 24 hours at 37°C in a CO2 incubator. After 24 hours, the medium was aspirated and Huh7 cells were washed with 0.5% BSA containing Hank’s balanced salt solution (bHBSS). The Huh7 cells were incubated with bHBSS (500 μL), 3.7 kBq carrier-free 125I (PerkinElmer Inc., Waltham, MA, USA), and 10 μM/L sodium iodide (NaI, specific activity of 740 MBq/mM) at 37°C for 30 minutes in a CO2 incubator. Huh7 cells were washed twice with chilled bHBSS, then lysed with 500 μL of 2% SDS. Then, radioactivity was measured using a Cobra-II gamma-counter (Canberra Packard, Mississauga, Canada). The uptake values were normalized with total protein determined by BCA protein assay kit (Thermo Fisher Scientific).
131I treatment and DNA damage assay
Huh7 (4×105) seeded cells were incubated with 20 mg/mL of EV-Huh7/NIS for 24 hours. The cells were washed with bHBSS and incubated with or without 50 μCi/mL 131I (KIRAMS, Seoul, Republic of Korea) supplemented with 30 μM NaI for 7 hours in a CO2 incubator. Cells were washed and re-seeded at a density of 1,000 cells/well in 8-well chamber slides. Cells were fixed with 4% paraformaldehyde after cells were attached to slides and blocked with 3% BSA in PBS. Cells were probed with anti-gamma H2A.X (phospho S139) antibody with Daylight 488 (Abcam; dilution: 1:200) overnight. Cells were washed with PBS (three times) and mounted using DAPI-containing mounting medium (Vector Laboratories, Burlingame, CA, USA). Images were attained with a Zeiss super-resolution confocal microscope (LSM 5 Exciter, Zeiss).
Statistical analysis
Data are presented as mean ± SD. The statistical significance was determined (Student’s t-test) by GraphPad Prism 7 software version 7.04 (GraphPad Software, Inc., La Jolla, CA, USA). A P-value less than 0.05 was considered to indicate statistically significant differences.
Results
Establishment of double-gene expression in Huh7 cells
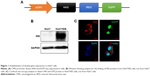
The construct was designed to transfer both hNIS and EGFP using an IRES (Figure 1A). The two genes were effectively expressed in Huh7 cells and called Huh7/NIS. Western blotting analysis showed overexpression of NIS protein in Huh7/NIS cells, but the protein was not detected in Huh7 cells (Figure 1B). NIS and EGFP expressions were further confirmed by fluorescence microscopy (Figure 1C). These results confirm the successful insertion and expression of hNIS gene in Huh7 cells.
Isolation and characterization of EVs
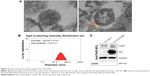
EVs were isolated from Huh7/NIS cells by ultracentrifugation as described previously. To confirm the typical morphology of EV-Huh7/NIS, the isolated EVs were analyzed using TEM. EV-Huh7/NIS exhibited a spherical morphology with an approximate diameter of 150 nm (Figure 2A). Furthermore, the TEM images (right panel) showed a clear bi-lipid layer of EVs (Figure 2A). ELS was used to analyze the size of EV-Huh7/NIS, and revealed that EV-Huh7/NIS were, on average, 169.2±71.5 nm in size and ranged from 50–450 nm (Figure 2B). Furthermore, ELS also revealed that the Zeta potential of EV-Huh7/NIS was approximately −2.20 mV (Figure 2B). Isolated EVs from Huh7 and Huh7/NIS cells were subjected to Western blot to confirm the positive (CD63, an EV-associated membrane protein) and negative (GM130, a Golgi apparatus protein) markers, and the results demonstrated the presence of CD63 in the EVs isolated from both the cells, whereas, GM130 was absent in EVs and present in whole cell lysates (Figure 2C). Together, these results suggested successful isolation of EVs from the cells.
NIS protein is secreted via EVs isolated from Huh7/NIS
To transfer the NIS protein to another cell, EVs should possess the NIS protein. EV-Huh7 and EV-Huh7/NIS lysates were subjected to Western blotting. The results revealed a high amount of NIS protein in EV-Huh7/NIS but no corresponding band was detected in EV-Huh7 (Figure 2C). Taken together, these data provide an indication that EV-Huh7/NIS contained NIS protein. This result paved the way for the next step, which was transferring NIS protein to other cells that expressed no or negligible NIS protein.
EV-mediated transfer of NIS protein to cells
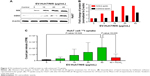
Here, we attempted to determine whether NIS-comprising EVs’ internalization leads to increase in the NIS protein in recipient cells (Huh7 cells). Huh7 parent cells were incubated with EV-Huh7/NIS (5, 10, 15, and 40 μg/mL) for 24 hours. Western blotting results revealed increases in the levels of NIS protein in the recipient Huh7 cells in a dose-dependent manner (Figure 3A and B). Transfer of the protein from EVs to another cell was further confirmed by CD63 expression in Huh7 cells, which increased in a dose-dependent pattern similar to that of the NIS protein (Figure 3A and B). These data suggested that NIS protein was successfully transferred to recipient cells by EVs.
Transfer of NIS protein leads to enhanced 125I uptake in vitro
To confirm that active and functional NIS proteins were transferred to the recipient Huh7 cells, EV-Huh7/NIS (5, 10, 15, and 40 μg/mL) were incubated with Huh7 cells for 24 hours and subjected to in vitro 125I uptake assay. Results revealed that 125I uptake was raised in a dose-dependent manner in recipient Huh7 cells. Cells incubated with EV-Huh7/NIS (40 μg/mL) showed a significant (P=0.026) increase in the level of 125I uptake compared to control cells. Furthermore, 125I uptake was successfully blocked (P=0.036) by addition of KClO4 (a known blocker of NIS-mediated 125I uptake) (Figure 3C). These results revealed that the transferred NIS proteins were active and functional.
EV-Huh7-NIS followed by 131I treatment leads to increased DNA damage
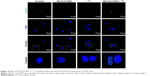
To evaluate whether EV-Huh7-NIS treatment enhances the therapeutic effect of 131I, cells were pre-treated with EV-Huh7-NIS, followed by 131I exposure, immunofluorescent assay for γH2A.X (DNA double strand breaks [DSB]) marker was performed. The results revealed that no DSB was observed in control and EV-Huh7-NIS-treated cells. A slight DSB was observed in 131I only treatment, but combination treatment of EV-Huh7-NIS and 131I showed highest DSB among all other treatments (Figure 4).
Discussion
NIS has the benefit of having both reporter gene and therapeutic functions, therefore, NIS gene transfer makes it possible to visualize, monitor, and treat tumors with suitable radionuclides.16 Several preclinical studies have made an effort to increase the NIS levels in thyroid cancer cells with pharmacological interventions, with partial success.17–19 Other studies have investigated intonation of endogenous, efficient NIS expression by histone deacetylase inhibitors in breast cancer.20,21 Another study with cabozantinib and sorafenib demonstrated blockade of the MAPK pathways leading to an increase in iodine uptake in papillary thyroid cancer cells.22 Nevertheless, multiple tyrosine kinase inhibitors decreased patients’ tolerability compared to BRAF/MEK inhibitors.23,24 Therefore, a novel strategy is warranted for the modulation of expression of NIS in thyroid and non-thyroid cancers.
EVs can transport various biological materials for short and long distances within a biological system. Numerous studies have shown that EVs are able to transfer biologically active proteins (such as VEGF, CLIC1, and EBAG9) from one cell to another.7,25,26 We proposed to elucidate the nature of EV-mediated protein transfer from one cell to another by transferring NIS protein to cancer cells exhibiting no or low NIS expression. In the present study, we experimented with a human HCC cell line (Huh7). We successfully constructed the HCC cell line which expressed two genes, NIS and EGFP, driven by a single promoter. The EGFP gene simplifies fluorescence in vitro imaging, in addition, in vitro cell sorting was performed by FACS analysis. We confirmed the translation of the NIS and EGFP gene by Western blotting and immunofluorescence imaging, which is consistent with our previous studies.4,6
The presence of CD63, an EV biomarker protein, and the absence of Golgi apparatus marker protein confirmed that EV preparations were proficiently enriched from Huh7/NIS cell culture supernatant. The EV-Huh7/NIS displayed a characteristic round shape and intact with a mean diameter of 169.2±71.5 nm (range 50–450 nm). Our results confirmed that isolated EV-Huh7/NIS were cell-derived, as reported previously.9,15
Radioiodine treatment is generally effective for patients with thyroid cancer, nonetheless it has turned out to be ineffective where thyroid cancer cells lose NIS expression and do not respond to radioiodine.3,27 Low or no NIS expressing tumors such as melanoma, liver cancer, and gastric cancer can be non-responsive to radioiodine.21 Therefore, introducing NIS protein could improve therapeutic effects of radiotherapy in such tumors. So, we used EVs to transport NIS protein to tumor cells expressing low/no NIS. Here, we took HCC as our target tumor. EVs isolated from the Huh7/NIS cells exhibited high levels of NIS protein, which is essential for transfer of the protein to recipient cells, and they have already been shown to transport active and functional proteins to recipient cells.7,25,26
Further, we transported the NIS protein to Huh7 recipient cells by means of EVs. Incubated EVs enriched with NIS protein transferred to recipient Huh7 cells led to an increase in NIS protein levels in cells. Further, we confirmed that the transportation of protein to recipient cells was EV-mediated, by analyzing the CD63 levels after the transfer of EVs enriched with NIS protein to recipient Huh7 cells, which showed a dose-dependent increase in the CD63 levels. This further confirmed the successful transportation of NIS protein to recipient cells. It was important to test whether the transported proteins were active and biologically functional. Therefore, we performed the 125I uptake assay in parent Huh7 cells treated with EVs enriched with NIS protein. Our results showed that the treatment substantially increased 125I uptake in a dose-dependent manner. This result confirms the functional aspects of the NIS protein transported via EVs. Furthermore, KClO4 treatment blocked the 125I uptake in the recipient cells, which confirmed the 125I uptake was solely due to enhanced levels of NIS protein in the recipient cells. The 131I treatment of Huh7 cells pretreated with EV-Huh7/NIS increased γH2A.X subnuclear accumulation, indicating fatal and irreparable double stranded DNA breaks, which is an indicator of cell cytotoxicity.28 These initial results validate the further study of EVs as an NIS transfer platform for radioiodine therapies for radioiodine-resistant cancers and non-NIS expressing or reduced NIS-expressing cancer cells.
Despite the novel and advanced findings of this study, there are still several challenges which need to be addressed. First, regarding the production of EVs on a large scale for clinical use, recent studies have shown production of EVs on a large scale is possible by engineering extracellular vesicle-mimetic nanovesicles (ENV).29,30 We also recently showed more than 100-fold production of ENVs compared to EVs from the same number of cells from mesenchymal stem cells and red blood cells.31,32 Similarly, the ENVs can be produced from NIS-expressing cells on a large scale. In addition to our in vitro study, in vivo small animal studies are needed to evaluate efficiency of the EV-mediated transport of NIS protein. The biodistribution of EVs in tumor-bearing mice need to be studied. The non-specific transfer of EVs to other organs can be modulated by surface engineering, and tumors can be specifically targeted by adding targeting peptides or proteins to the EVs.31,33 Recently, EVs have been investigated more as drug delivery vehicles.8 EVs enriched with NIS protein can also be used to transfer anti-cancer drugs, which could further improve cancer therapies.
Conclusion
We successfully isolated EVs containing NIS protein from the cells expressing NIS. Further, we successfully transferred active NIS protein using EVs, which led to enhanced 125I uptake in recipient cells. Increased DSB in Huh7 cells through 131I treatment with EV-Huh7/NIS pretreatment confirms the increased cytotoxicity effect of EV-Huh7/NIS. The NIS-enriched EVs showed great efficiency in transferring NIS protein to cells, and such EVs can revert radioiodine-resistant cancers into radioiodine-sensitive cancers.
Acknowledgments
We would like to thank Ms Ji Min Oh, Dr Ho Won Lee, Mrs Ramya Lakshmi Rajendran, Mr Se Hwan Baek, Dr Senthilkumar Kalimuthu, Ms Liya Zhu, Prof Shin Young Jeong, Prof Sang-Woo Lee, and Prof Jaetae Lee for their help in performing the experiment and/or acquiring the fund. This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2016).
Disclosure
The authors report no conflicts of interest in this work.
References
Dohán O, De La Vieja A, Paroder V, et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24(1):48–77. | ||
Ahn B-C. Sodium iodide symporter for nuclear molecular imaging and gene therapy: from bedside to bench and back. Theranostics. 2012;2(4):392–402. | ||
Oh JM, Kalimuthu S, Gangadaran P, et al. Reverting iodine avidity of radioactive-iodine refractory thyroid cancer with a new tyrosine kinase inhibitor (K905-0266) excavated by high-throughput NIS (sodium iodide symporter) enhancer screening platform using dual reporter gene system. Oncotarget. 2018;9(6):7075–7087. | ||
Lee YL, Lee YJ, Ahn SJ, et al. Combined radionuclide-chemotherapy and in vivo imaging of hepatocellular carcinoma cells after transfection of a triple-gene construct, NIS, HSV1-sr39tk, and EGFP. Cancer Lett. 2010;290(1):129–138. | ||
Seidlin SM, Marinelli LD, Oshry E. Radioactive iodine therapy: effect on functioning metastases of adenocarcinoma of the thyroid. CA Cancer J Clin. 1990;40(5):299–317. | ||
Kim JE, Hwang MH, Lee HW, Lee SW, Lee J, Ahn BC. Combined RNA interference of adenine nucleotide translocase-2 and ganciclovir therapy in hepatocellular carcinoma. Nucl Med Biol. 2013;40(8):987–993. | ||
Gangadaran P, Rajendran RL, Lee HW, et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release. 2017;264:112–126. | ||
Gangadaran P, Hong CM, Ahn B-C. An Update on in Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front Pharmacol. 2018;9:169. | ||
Gangadaran P, Hong CM, Ahn B-C. Current perspectives on in vivo noninvasive tracking of extracellular vesicles with molecular imaging. Biomed Res Int. 2017;2017(8):1–11. | ||
Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol. 2008;180(2):817–824. | ||
Gasser O, Hess C, Miot S, Deon C, Sanchez J-C, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285(2):243–257. | ||
Lösche W, Scholz T, Temmler U, Oberle V, Claus RA. Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils. Platelets. 2004;15(2):109–115. | ||
Pluskota E, Woody NM, Szpak D, et al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112(6):2327–2335. | ||
Zhu L, Oh JM, Gangadaran P, et al. Targeting and therapy of glioblastoma in a mouse model using exosomes derived from natural killer cells. Front Immunol. 2018;9:824. | ||
Gangadaran P, Li XJ, Lee HW, et al. A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget. 2017;8(66):109894–109914. | ||
Chung J-K. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43(9):1188–1200. | ||
Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95(2):820–828. | ||
Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121(12):4700–4711. | ||
Cheng L, Jin Y, Liu M, Ruan M, Chen L. HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E. Oncotarget. 2017;8(12):19843–19854. | ||
Kelkar MG, Senthilkumar K, Jadhav S, Gupta S, Ahn BC, De A. Enhancement of human sodium iodide symporter gene therapy for breast cancer by HDAC inhibitor mediated transcriptional modulation. Sci Rep. 2016;6:19341. | ||
Liu Z, Xing M. Induction of sodium/iodide symporter (NIS) expression and radioiodine uptake in non-thyroid cancer cells. PLoS One. 2012;7(2):e31729. | ||
Ruan M, Liu M, Dong Q, Chen L. Iodide- and glucose-handling gene expression regulated by sorafenib or cabozantinib in papillary thyroid cancer. J Clin Endocrinol Metab. 2015;100(5):1771–1779. | ||
Cabanillas ME, Patel A, Danysh BP, Dadu R, Kopetz S, Falchook G. BRAF inhibitors: experience in thyroid cancer and general review of toxicity. Horm Cancer. 2015;6(1):21–36. | ||
Dadu R, Devine C, Hernandez M, et al. Role of salvage targeted therapy in differentiated thyroid cancer patients who failed first-line sorafenib. J Clin Endocrinol Metab. 2014;99(6):2086–2094. | ||
Miyazaki T, Ikeda K, Sato W, Horie-Inoue K, Inoue S. Extracellular vesicle-mediated EBAG9 transfer from cancer cells to tumor microenvironment promotes immune escape and tumor progression. Oncogenesis. 2018;7(1):7. | ||
Setti M, Osti D, Richichi C, et al. Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth. Oncotarget. 2015;6(31):31413–31427. | ||
Arturi F, Russo D, Schlumberger M, et al. Iodide symporter gene expression in human thyroid tumors. J Clin Endocrinol Metab. 1998;83(7):2493–2496. | ||
Chatterjee S, Thaker N, De A. Combined 2-deoxy glucose and metformin improves therapeutic efficacy of sodium-iodide symporter-mediated targeted radioiodine therapy in breast cancer cells. Breast Cancer. 2015;7:251–265. | ||
Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. | ||
Wu JY, Ji AL, Wang ZX, et al. Exosome-Mimetic Nanovesicles from hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci Rep. 2018;8(1):2471. | ||
Gangadaran P, Hong CM, Oh JM, et al. In vivo Non-invasive Imaging of Radio-Labeled Exosome-Mimetics Derived From Red Blood Cells in Mice. Front Pharmacol. 2018;9:817. | ||
Kalimuthu S, Gangadaran P, Rajendran RL, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. | ||
Gunassekaran GR, Hong C-M, Vadevoo SMP, et al. Non-genetic engineering of cytotoxic T cells to target IL-4 receptor enhances tumor homing and therapeutic efficacy against melanoma. Biomaterials. 2018;159:161–173. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.