Back to Journals » Cancer Management and Research » Volume 11
A Novel Scoring System for Patients with Recurrence of Hepatocellular Carcinoma After Undergoing Minimal Invasive Therapies
Authors Wang Q , Ma L , Li J, Yuan C , Sun J , Li K, Qin L, Zang C , Zhao Y, Zhao Y, Zhang Y
Received 25 July 2019
Accepted for publication 8 December 2019
Published 20 December 2019 Volume 2019:11 Pages 10641—10649
DOI https://doi.org/10.2147/CMAR.S224711
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Qi Wang,1 Liang Ma,2 Jianjun Li,2 Chunwang Yuan,2 Jianping Sun,1 Kang Li,1 Ling Qin,1 Chaoran Zang,1 Yanan Zhao,1 Yan Zhao,3,* Yonghong Zhang1,2,*
1Research Center for Biomedical Resources, Beijing You’an Hospital, Capital Medical University, Beijing 100069, People’s Republic of China; 2Interventional Therapy Center for Oncology, Beijing You’an Hospital, Capital Medical University, Beijing 100069, People’s Republic of China; 3Clinical Detection Center, Beijing You’an Hospital, Capital Medical University, Beijing 100069, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghong Zhang
Research Center for Biomedical Resources; Interventional Therapy Center for Oncology; Beijing You’an Hospital, Capital Medical University, 8 Xitoutiao, Youanmenwai Street, Fengtai District, Beijing, People’s Republic of China
Tel +86 138 1010 8505
Email [email protected]
Yan Zhao
Clinical Detection Center Beijing You’an Hospital, Capital Medical University, 8 Xitoutiao, Youanmenwai Street, Fengtai District, Beijing, People’s Republic of China
Tel +86 189 1138 0390
Email [email protected]
Background: The higher recurrence rate of hepatocellular carcinoma (HCC) needs to be urgently controlled. However, definitive markers are lacking for patients with recurrence of HCC after undergoing minimal invasive therapies—local ablation combined with transcatheter arterial chemoembolization (TACE).
Materials and methods: Demographic and clinicopathological data of 234 subjects receiving combined therapies as the initial treatment were retrospectively analyzed. Univariate and multivariate Cox regression analysis was used to assess independent risk factors of recurrence. Selected variables were divided into low-, intermediate-, and high-risk groups of recurrence according to the scores assigned to them based on their respective hazard ratio (HR) values. The area under the curve (AUC) was used to evaluate the predictive value of the scoring system. Cumulative recurrence-free survival (RFS) and overall survival rates were calculated by the Kaplan–Meier estimator. Finally, a correlation analysis was performed on demographic and clinical data among the three groups.
Results: The AUC of predicting 1-, 2-, and 3-year recurrence rates was 0.680, 0.728, and 0.709, respectively. The cumulative RFS rate in the low-risk group at 1, 2, and 3 years after undergoing combined treatments was 4%, 12.2%, and 30.6%, while that in the intermediate-risk group and high-risk group was 23.4%, 51.6%, 60.0%, and 47.3%, 78.2%, 83.6%, respectively. Gamma-glutamyltransferase (γ-GT), blood urea nitrogen (BUN), and total cholesterol (TC) levels among the three groups were statistically different.
Conclusion: The scoring system of the present study for patients with the recurrence of HCC after undergoing TACE combined with local ablation may help physicians make a reasonable clinical decision, providing ideal management for diagnosis and treatment.
Keywords: ablation, hepatocellular carcinoma, transcatheter arterial chemoembolization, scoring system, recurrence
Introduction
Hepatocellular carcinoma (HCC), as the sixth most common malignancy and the fourth tumor-related death worldwide, is a global health problem, especially in developing countries.1 In today’s clinical practice, the higher recurrence rate is one of the leading causes of mortality, negatively influencing the long-term prognosis of patients with HCC.2,3 Thus, monitoring for recurrence and providing timely treatment might improve the prognosis of patients with HCC.4
Minimal invasive therapies such as local ablation coupled with transcatheter arterial chemoembolization (TACE) have been an alternative treatment for patients with poor liver function, fewer liver donors, and bad economic conditions.5,6 This could be explained as follows: (1) TACE can label a tumor and cut off its blood supply by blocking the hepatic artery, leaving the tumor starved; (2) TACE can enhance the therapeutic potential of locoregional ablation.7–9
Over the last few years, numerous scholars have devoted themselves to studying some markers that are associated with the early recurrence of patients with HCC after TACE or curative ablation therapy, including tumor number, fibrinogen (Fib), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and so on.10,11 Nevertheless, relevant studies on combined therapies are lacking.
Therefore, the objective of this study was to establish a novel scoring system based on clinicopathological data of patients who underwent combined therapies as the initial treatment. It was assumed that the system might effectively assess the risk of recurrence and help clinicians adjust the follow-up strategy, administer timely anti-tumor treatment to patients, and eventually improve their prognosis.
Materials and Methods
Subjects of Study
From January 1, 2015, to December 31, 2016, a total of 305 subjects who were newly diagnosed with HCC were initially treated with combined therapies in the Beijing You’an Hospital. The diagnosis of HCC was completed based on the radiological/histological criteria proposed by the guidelines of the American Association for the Study of Liver Disease.12 This study’s inclusion criteria were as follows: (1) Age from 18 to 75 years, (2) TACE combined with locoregional ablation as the initial anticancer treatment, and (3) no other malignancies that may affect the prognosis. The exclusion criteria were as follows: (1) Radiological evidence of invasion into the major portal/hepatic vein branches, (2) the presence of extrahepatic metastases, (3) severe coagulation disturbance, and (4) secondary liver cancer. The study protocol, conformed to the 1990 Declaration of Helsinki, was approved by the Ethics Committee of the Beijing You’an Hospital.
Data collected were as follows: demographic data (age, sex, history of hypertension, and diabetes mellitus), etiologies of HCC [hepatitis B virus (HBV), hepatitis C virus (HCV), and alcoholic liver disease], tumor-related indices [number of tumors, size of tumors, and alpha-fetoprotein (AFP) level], liver function indices [cirrhosis, Child-Pugh class, and alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and gamma-glutamyl transferase (γ-GT) levels], routine blood examinations [(neutrophil count, lymphocyte count, platelet count, and Fib and albumin-to-prealbumin ratio (APR) levels)], ablation modalities (radiofrequency ablation, microwave ablation, and argon-helium cryoablation).
TACE Procedure
The procedure was performed by three qualified hepatologists. A microcatheter was placed approximately in the tumor-feeding artery by the selective/superselective technique. A mixture of doxorubicin (Pfizer Inc., NY, USA) and lipiodol (Guerbet, Villepinte, France) was injected, and Gelfoam was used for embolization. Occlusions of the feeding artery and disappearance of the vessel stain were identified as the endpoint of embolization.
Ablation Procedure
Local ablation was performed by qualified hepatologists within 2 weeks after TACE. With the guidance of computed tomography (CT) or magnetic resonance imaging (MRI), the ablative position and modality were determined. Then anesthesia was injected at the puncture site, and the ablation needle was inserted into the skin. Multiple sites, overlapping ablation, and repeated ablation were considered according to the tumor number and size to achieve complete ablation. A safety margin of 0.5–1.0 cm of the adjacent non-neoplastic tissue was ablated to ensure complete coverage.
Follow-Up
All patients were followed-up at the outpatient clinic. Abdominal CT or MRI was carried out 4–6 weeks after the treatment to determine whether the ablation was complete. A salvage treatment was required until the ablation was complete. The follow-up involved the standard physical and blood examination every 3 months, ultrasonography every 3–6 months, and CT/MRI every 6 months.
If the CT/MRI displayed an enhancing area within, adjacent to, or out of the original neoplasms, recurrence would be recognized. The recurrence-free survival (RFS) was delineated as the time from the termination of treatments to the first record of a detectable relapse or the date of mortality without evidence of HCC-related recurrence. The overall survival (OS) was defined as the time from the termination of treatments to the date of HCC-related mortality or the data of the last follow-up in which the expiration date in the present study was April 1, 2019. Individuals were treated again with TACE or ablation when recurrence was confirmed. The data of patients were kept confidential. In the present study with minimum risk, the requirement for informed consent was waived because it was difficult to reconnect with the patient.
Statistical Analysis
Continuous and categorical variables, expressed as the mean ± standard deviation and frequency, were compared by the Student t-test and Chi-square test, respectively. The baseline γ-GT was compared by the Mann–Whitney U-test. The univariate and multivariate Cox proportional hazard model was used to identify independent risk factors of recurrence in patients with HCC receiving combined therapies. The optimal cutoff values were calculated in accordance with the time-dependent receiver operating characteristic curves and Youden’s index. The recurrence and survival curves among the three groups with different risks were generated by the Kaplan–Meier estimator and were compared by the log rank test. The prediction value of the scoring system was determined by the area under the curve (AUC). A P value less than 0.05 was regarded as statistically significant. All the data were processed by SPSS 25.0 (IBM, NY, USA).
Results
Patient Characteristics and Follow-Up results
A total of 71 subjects were excluded from the present study in line with the inclusion and exclusion criteria. The selection method is shown in Figure 1 in detail. Eventually, the clinicopathological data of 234 patients with HCC treated with combined therapies as the initial treatment were retrospectively analyzed. The present study included 182 male (77.8%) and 52 female (22.2%) patients. In addition, 64 patients (27.4%) had high blood pressure and 50 (21.4%) had type 2 diabetes mellitus. Further, 97 patients (41.5%) had a history of smoking and 75 (32.1%) had a history of drinking. For etiologies, 176 (75.2%) patients had HBV-related HCC, 43 (18.4%) had HCV-related HCC, and 15 (6.4%) had alcohol-related HCC (Table 1). The median follow-up was 38.2 months (25~75th percentiles, 36.0~43.3 months). A total of 143 (61.1%) patients were diagnosed with tumor recurrence, and 31 (13.2%) died of HCC. The cumulative recurrence rate of 1, 2, and 3 years was 24.4% (57/234), 48.7% (114/234), and 59.0% (138/234), and the corresponding OS rate was 98.3% (230/234), 94.0% (220/234), and 88.5% (207/234), respectively.
 |
Table 1 Demographic and Clinicopathological Data of HCC Patients |
 |
Figure 1 Patients selection process. |
Prognostic Factors Associated with HCC Recurrence
Based on whether relapses occurred within 2 years or not, all patients were divided into recurrence and non-recurrence groups. Subsequently, demographic and clinicopathological data of these two groups were compared. The results showed a statistical difference in some variables including the history of smoking, Child-Pugh class, size and number of tumors, AFP, γ-GT, Fib, BUN, and APR (Table 2). The variables with the P value less than 0.1 in Table 2 were analyzed by univariate and multivariate Cox regression analysis for identifying variables associated significantly with a poor RFS rate. Finally, the findings of this study suggested that gender, number of tumors, AFP, Fib, and APR were independent risk factors of HCC recurrence (Table 3).
 |
Table 2 The Comparison of Clinical Data Between Recurrence/Non-Recurrence Group |
 |
Table 3 Prognostic Factors for RFS by Cox Proportional Hazards Regression Model |
Establishment of the Scoring System
Each variable selected by multivariate analysis in Table 3 was assigned different scores according to their HR value. Men were assigned a score of 2; patients with multiple tumors were assigned a score of 2; patients with different AFP levels (<7 ng/mL, 7–400 ng/mL, and ≧400 ng/mL) were assigned scores of 0, 1, and 2, respectively; patients with different Fib levels (<3.105 mg/dL and ≧3.105 mg/dL) were assigned scores of 0 and 1, respectively; and patients with different APR levels (<0.250 and ≧0.250) were assigned scores of 0 and 4, respectively (Table 4). In conclusion, a scoring system was obtained, which ranged from 0 to 11 by calculating each patient’s score. Individuals with scores of 0–3 were defined to be at low recurrence risk, 4–7 at immediate recurrence risk, and 8–11 at high recurrence risk. The time-dependent AUC of 1 year, 2 years, and 3 years was 0.680 (95% CI: 0.604–0.756), 0.728 (95% CI: 0.664–0.793), and 0.709 (95% CI: 0.641–0.776), respectively (P < 0.0001).
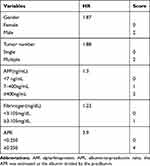 |
Table 4 The Assignment of Variables According HR |
Comparison of Clinicopathological Data Among the Three Groups with Different Recurrence Risks
The comparison of demographic and clinicopathological data revealed that some variables, such as history of smoking, Child-Pugh class B, high ALT, high AST, large size of tumors, and so on, representing the poor functional status of liver were significantly associated with a higher recurrence risk (Table 5). Especially, a higher γ-GT level and lower BUN and TC levels indicated a higher recurrence risk (Figure 2). And then, grouped by cut-off values of these three variables, the differences in the recurrence rates between the groups were statistically significant (Figure 3).
 |
Table 5 The Comparison of Clinical Data Among Groups with Different Recurrence Risk |
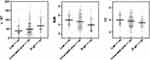 |
Figure 2 The association between γ-GT, BUN, and TC and different recurrence risk. Abbreviations: γ-GT, gamma-glutamyltransferase; BUN, blood urea nitrogen; TC, total cholesterol. |
 |
Figure 3 The cumulative recurrence risk in different levels of γ-GT, BUN, and TC. Abbreviations: γ-GT, gamma-glutamyltransferase; BUN, blood urea nitrogen; TC, total cholesterol. |
The 1-, 2-, and 3-year cumulative RFS rates of the low-risk group were 4%, 12.2%, and 30.6%, while those of the intermediate-risk group and high-risk group were 23.4%, 51.6%, 60.0% and 47.3%, 78.2%, 83.6%, respectively. Differences between groups were statistically significant. The 1-, 2-, and 3-year cumulative OS rates of the low-risk group were 99.0%, 99.0%, and 99.0%, while those of the intermediate-risk group and high-risk group were 100%, 95.2%, 91.1%, and 98.2%, 89.1%, 74.5%, respectively. A statistically significant difference was also observed between the groups (Figure 4).
 |
Figure 4 Kaplan-Meier survival curve of RFS and OS for patients with different recurrence risk. Abbreviations: RFS, recurrence-free survival; OS, overall survival. |
Discussion
The present study indicated that the 3-year OS rate of patients with HCC after receiving percutaneous ablation coupled with TACE reached 88.5%, suggesting that TACE is one of the effective alternative treatments for HCC. However, the therapeutic effect and quality of life of patients are influenced by the higher recurrence rate.2,3 The absence of accurate markers might deprive individuals with a high risk of relapse of access to timely interventions. Thus, valuable markers or models should be developed to improve prognosis of patients with HCC.
Many scholars are gradually focusing on demographic and laboratory data, which are secure, repeatable, and easily available for predicting the recurrence of patients with HCC receiving only TACE or ablation therapy. Various studies showed that several predictors might indicate poor RFS, some of which are as follows: (1) noninvasive fibrosis markers such as aspartate APR index and age-to-platelet index;13,14 (2) liver function markers such as albumin and albumin-to-bilirubin index;15,16 (3) tumor-related markers such as tumor number, AFP, lens culinaris agglutinin-reactive fraction of AFP-L3, and des-gamma-carboxy prothrombin;17–19 (4) inflammatory markers such as NLR, PLR, and prognostic nutritional index; and20,21 (5) pathological markers such as microvascular invasion and lowly-differentiated neoplasm.22,23
But little attention has been paid to the relationship between these indicators or combined markers and prognosis of patients with HCC treated with TACE plus percutaneous ablation. However, the present study illustrated the development of a novel scoring system based on gender, tumor number, AFP, Fib, and APR selected by univariate and multivariate analysis, which might evaluate the recurrence risk of patients with HCC after undergoing combined therapies.
First, the present study showed that men had a higher risk of relapse compared with women, which could be explained by two points: androgen receptor activation might contribute to HCC cell progression and invasion, and the endogenous metabolite of estrogen could suppress tumor growth because of its antiproliferative, proapoptotic, and antiangiogenic activities.24,25 Second, multiple tumors are the characteristic of multicentric development, which could not be usually detected by an imaging test, with a higher possibility to relapse and metastasize Third, AFP, an independent risk factor for recurrence in the present study, might be an indication of vascular invasion and HCC progression, contributing to an increased risk for early recurrence and is in agreement with Yang’s conclusion.26,27 Then, deposited Fib in tumor tissue, as an extracellular matrix, could promote adhesion and migration of tumor cells by contributing to the angiogenesis of tumors.28 Finally, APR, a new combination of albumin and prealbumin, perhaps was explained by two viewpoints: Supplemental infusion of albumin or blood transfusion might make exogenous albumin persist in the body for several days as a consequence of a long half-life of 20 days;29 prealbumin, as a sensitive marker to reflect protein synthesis and secretion owing to a short half-life of 0.5 day, might predict the recurrence risk of HCC.30
In this study, discrepancies were found in the BUN and TC levels among the groups with different risks of relapse. Therefore, it was considered that abnormality in protein and lipid metabolism among the three groups might be associated with the recurrence of HCC. Further research would be undertaken to confirm these ideas.
Few studies have explored the prognostic markers of patients with HCC after undergoing TACE plus ablation therapies. This study presented a risk scoring system based on APR, a novel marker. However, confounders related to the retrospective study could not be avoided, and parameters such as NLR, PLR, and other newer predictors were not investigated. Thus, further prospective studies including more markers are needed to be carried out.
Conclusion
In conclusion, the novel scoring system could help stratify patients with HCC into groups with different recurrence risks, following which a personalized surveillance strategy and timely antineoplastic therapies could be implemented.
Acknowledgment
The authors highly appreciate all the patients who were involved in the present study and our team from Beijing You’an Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Heimbach JK. Overview of the updated AASLD guidelines for the management of HCC. Gastroenterol Hepatol. 2017;13(12):751–753.
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
4. Benson AB
5. Borie F, Bouvier A-M, Herrero A, et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol. 2008;98(7):505–509. doi:10.1002/jso.v98:7
6. Kim AR, Park E, Kwon SY, et al. Efficacy and safety of combined radiofrequency ablation with transarterial chemoembolization in patients with barcelona clinic liver cancer stage a hepatocellular carcinoma ineligible for curative treatment. Korean J Gastroenterol. 2019;73(3):167–176. doi:10.4166/kjg.2019.73.3.167
7. Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116(23):5452–5460. doi:10.1002/cncr.25314
8. Ahrar K, Newman RA, Pang J, Vijjeswarapu MK, Wallace MJ, Wright KC. 2004 Dr. Gary J. Becker Young investigator award: relative thermosensitivity of cytotoxic drugs used in transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2004;15:901–905. doi:10.1097/01.RVI.0000136829.36643.ED
9. Iezzi R, Pompili M, La Torre MF, et al. Radiofrequency ablation plus drug-eluting beads transcatheter arterial chemoembolization for the treatment of single large hepatocellular carcinoma. Dig Liver Dis. 2015;47:242–248. doi:10.1016/j.dld.2014.12.007
10. Huang G, Jiang H, Lin Y, et al. Prognostic value of plasma fibrinogen in hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;29;10(Oct):5027–5041. doi:10.2147/CMAR.S175780
11. Chen K, Zhan MX, Hu BS, et al. Combination of the neutrophil to lymphocyte ratio and the platelet to lymphocyte ratio as a useful predictor for recurrence following radiofrequency ablation of hepatocellular carcinoma. Oncol Lett. 2018;15(1):315–323. doi:10.3892/ol.2017.7291
12. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
13. Chung HA, Kim JH, Hwang Y, et al. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J Gastroenterol. 2016;22(1):57–63. doi:10.4103/1319-3767.173760
14. Seo JY, Kim W, Kwon JH, et al. Noninvasive fibrosis indices predict intrahepatic distant recurrence of hepatitis B-related hepatocellular carcinoma following radiofrequency ablation. Liver Int. 2013;33(6):884–893. doi:10.1111/liv.12132
15. Sheng RF, Zeng MS, Ren ZG, et al. Intrahepatic distant recurrence following complete radiofrequency ablation of small hepatocellular carcinoma: risk factors and early MRI evaluation. Hepatobiliary Pancreat Dis Int. 2015;14(6):603–612. doi:10.1016/S1499-3872(15)60390-3
16. Oh IS, Sinn DH, Kang TW, et al. Liver function assessment using albumin-bilirubin grade for patients with very early-stage hepatocellular carcinoma treated with radiofrequency ablation. Dig Dis Sci. 2017;62(11):3235–3242. doi:10.1007/s10620-017-4775-8
17. Tateishi R, Shiina S, Yoshida H, et al. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology. 2006;44(6):1518–1527. doi:10.1002/(ISSN)1527-3350
18. Lee S, Rhim H, Kim YS, et al. Post-ablation des-gamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int. 2016;36(4):580–587. doi:10.1111/liv.12991
19. Douhara A, Namisaki T, Moriya K, et al. Predisposing factors for hepatocellular carcinoma recurrence following initial remission after transcatheter arterial chemoembolization. Oncol Lett. 2017;14(3):3028–3034. doi:10.3892/ol.2017.6489
20. Chu MO, Shen CH, Chang TS, et al. Pretreatment inflammation-based markers predict survival outcomes in patients with early stage hepatocellular carcinoma after radiofrequency ablation. Sci Rep. 2018;8(1):16611. doi:10.1038/s41598-018-34543-z
21. Hu J, Wang NY, Yang YF, et al. Diagnostic value of alpha-fetoprotein combined with neutrophil-to-lymphocyte ratio for hepatocellular carcinoma. BMC Gastroenterol. 2018;18(1):186. doi:10.1186/s12876-018-0908-6
22. Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol. 2015;27(3):349–354. doi:10.1097/MEG.0000000000000270
23. Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2019.
24. Ao JP, Meng J, Zhu L, et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012;6(5):507–515. doi:10.1016/j.molonc.2012.06.005
25. Du B, Wang SY, Shi XF, et al. The effect of 2-methoxyestradiol liposome on growth inhibition, angiogenesis and expression of VEGF and Ki67 in mice bearing H22 hepatocellular carcinoma. Tumori. 2011;97(5):660–665. doi:10.1177/030089161109700520
26. Yang SL, Liu P, Yang S, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(6):716–724. doi:10.1002/bjs.10093
27. Gomaa AI, AI-Khatib A, Abdel-Razek W, et al. Ascites and alpha-fetoprotein improve prognostic performance of Barcelona clinic liver cancer staging. World J Gastroenterol. 2015;21(18):5654–5662. doi:10.3748/wjg.v21.i18.5654
28. Garcia MG, Bayo J, Bolontrade MF, et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011;8(5):1538–1548. doi:10.1021/mp200137c
29. Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–255. doi:10.2147/IJGM.S102819
30. Shenkin A. Serum prealbumin: is it a marker of nutritional status or of risk of malnutrition? Clin Chem. 2006;52(12):2177–2179. doi:10.1373/clinchem.2006.077412
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
