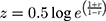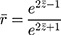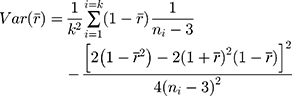Back to Journals » Medical Devices: Evidence and Research » Volume 13
A Novel Radiofrequency Device to Monitor Changes in Pulmonary Fluid in Dialysis Patients
Authors Connaire JJ, Sundermann ML, Perumal R, Herzog CA
Received 15 August 2020
Accepted for publication 28 October 2020
Published 11 November 2020 Volume 2020:13 Pages 377—383
DOI https://doi.org/10.2147/MDER.S277159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jeffrey J Connaire,1 Matthew L Sundermann,2 Ramu Perumal,2 Charles A Herzog3
1Davita Clinical Research, Minneapolis, MN, USA; 2ZOLL, Pittsburgh, PA, USA; 3Cardiology Division, Department of Internal Medicine, Hennepin Healthcare/University of Minnesota, Minneapolis, MN, USA
Correspondence: Jeffrey J Connaire
DaVita Clinical Research, 825 S 8 th St, Suite 300, Minneapolis, MN 55404, USA
Tel +1 612 202-1581
Fax +1 866 852-3241
Email [email protected]
Background and Objectives: Fluid monitoring is an important management strategy in patients with chronic kidney disease (CKD) and heart failure (HF). The μCor™ Heart Failure and Arrhythmia Management System uses a radiofrequency-based thoracic fluid index (TFI) to track pulmonary edema. During hemodialysis, the acute removal of fluid through ultrafiltration offers a model for measuring a patient’s fluid status. The objective of the study was to assess the relationship between the device measured TFI and ultrafiltration volume (UFV).
Design, Setting, Participants, and Measurements: Patients undergoing chronic dialysis with and without heart failure were enrolled in the study. The relationship between TFI and UFV in each individual subject was assessed by calculating the Pearson correlation coefficient (r). The average correlation across all subjects was calculated through the use of the Fisher’s z transform. Responder analysis was performed to assess the magnitude of change in TFI before and after dialysis.
Results: Twenty subjects were enrolled in the trial. The mean volume of fluid removal was 3.63 L (SD 0.88 L). The mean correlation based on Fisher’s transform was 0.95 CI (0.92– 0.99). Responder analysis showed that the mean reduction of TFI after dialysis was 5.5% ± 3.8.
Conclusion: The μCor system provides radiofrequency-based measurements of thoracic fluid which correlate well with total body fluid removal in a real-world setting. Fluid management based on the radar-derived TFI may provide benefits to dialysis patients and serves as a potential model for pulmonary edema common to the clinical course of heart failure.
Keywords: heart failure, total body fluid monitoring, dialysis, wearables, ultrafiltration
Introduction
Fluid monitoring is an important management strategy in patients with chronic kidney disease (CKD) and heart failure (HF).1,2 Assessment of fluid status changes in these patients is especially important when introducing therapies that target diuresis or fluid removal, and serial fluid measurements are often required to allow for non-invasive fluid monitoring to capture temporal trends. Symptomatic response to fluid removal in patients with both CKD and HF presents a unique classification challenge where fluid assessment before and after ultrafiltration (UFV) offers context to the underlying disease.3 Furthermore, measurement of lung fluid using radiofrequency (RF) measurements may serve as a viable option to remotely monitor patients with heart failure, and RF when delivered at the optimum frequency range offers fluid detection capabilities in the lungs.4 Detection of early fluid changes in CKD and HF patients may potentially assist with remote patient management.
Wearable sensors are promising tools for fluid monitoring and have the potential to improve patient outcomes.5 Recent studies of fluid monitoring in the clinical setting have involved point-of-care approaches such as lung ultrasonography (LUS) or device measured bioimpedance to aid in the diagnosis and prognosis of fluid accumulation.6,7 A wearable sensor capable of taking serial RF measurements would be a valuable tool for the clinician as fluid status could then be assessed through temporal trends in RF measurements.
Clinically, serial fluid status evaluation is most often required when rapid changes in fluid occur in settings such as hemodialysis, as well as in clinical settings such as heart failure where pulmonary edema worsens over time due to acute disease exacerbations. Fluid removal during hemodialysis offers a unique opportunity to assess acute fluid changes since a liter or more of fluid is removed over a short period of time. Furthermore, a previous study of 75 hemodialysis patients demonstrated that 63% of the study’s subjects were shown to have moderate to severe lung congestion before UFV began.8
In this study, the performance of the µCor system, a non-invasive wearable monitor that assesses fluid change through RF measurements, was evaluated in the context of hemodialysis. The µCor system consists of a patch-based sensor and is designed to non-invasively record, store, and transmit physiological data to medical professionals. The µCor system records an RF-derived measurement termed thoracic fluid index (TFI), electrocardiogram, heart rate, respiration rate, activity, and posture. Measurements acquired by the µCor system are transmitted wirelessly to a remote server with dedicated software for processing.
The primary objective of the study was to assess the correlation of TFI measurements taken by the µCor system to fluid removal within dialysis patients. Our hypothesis was that the µCor system would accurately track total body fluid removal with RF measurements in dialysis subjects.
Materials and Methods
The study was designed as a prospective and non-significant risk study. Subjects were informed of the purpose of the trial, were required to provide signed informed consent prior to any study-related procedures, and were informed that the trial was conducted in accordance with the Declaration of Helsinki. The study was IRB approved (IntegReview). Subjects included in the study had to be at least 21 years of age, had to be scheduled to undergo hemodialysis 3 times a week, had to have had ongoing hemodialysis for at least 3 months, and had to have been prescribed a net fluid removal of at least 2.5 L. The trial protocol and summary results are available under NCT03072732 at ClinicalTrials.gov, and the authors have direct access to the primary data from the trial. Additional data requests will not be granted due to the proprietary nature of the study device. The amount of fluid removal on the day of each test was determined per standard of care. Subjects underwent one hemodialysis session at one of the three separate hemodialysis sites.
µCor System
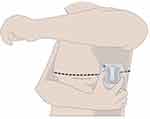
The study device shown in Figure 1, commercially available as the µCor™ Heart Failure and Arrhythmia Management System, is an adhesive wearable sensor that uses RF reflections to establish TFI. The device’s antenna emits RF signals which propagate through the lungs where signals are then reflected back to the device. Changes in radar wave signal path duration and strength indicate changes in fluid accumulation; the waves are highly modulated by tissue hydration. RF reflections are then used to calculate TFI, a relative index that is normalized to a baseline. Valid TFI measurements are distinguished from invalid TFI measurements through device thresholds including activity level measured through an accelerometer on the device sensor, RF signal quality, and sensor position. The accelerometer is set so that TFI measurements are not taken during a maximum threshold of activity as well as within specific pitch and roll ranges. The TFI validity requirements ensure that readings are taken during consistent conditions. The µCor system’s intended use is in outpatient clinics and home settings.
Study Procedure
At the clinical baseline, the subject’s medical history, weight, blood pressure, and heart rate were recorded. Subjects were placed in the dialysis chair per standard of care throughout the duration of the study. To optimize measurements, subjects were instructed to maintain the same posture throughout fluid removal as much as possible.
RF readings were recorded during dialysis with the µCor device placed in the left midaxillary position. The µCor device was programmed to record measurements every 3 min (±1 min) during the hemodialysis session, including at least 15 min before the start of the hemodialysis session through at least 15 min after the end of the hemodialysis session. During the course of the hemodialysis session, automated measurements of the UFV (in mL) were obtained from the dialysis machine every 6 min (±1 min). These UFV measurements were time-synchronized with the TFI readings within 1.5 min.
After 15-min post-dialysis, the µCor device was removed and subject weight, blood pressure, and heart rate were recorded. The oral intake of any fluids or solids and the volume of intravenous (IV) fluids administered during the hemodialysis session were recorded. The volume of urine output during the hemodialysis session also was recorded. Adverse events were collected from the time of enrollment through the end of the study.
Data Analysis
Pearson’s correlation coefficients were calculated for UFV and TFI for each individual subject. To determine the average correlation across all subjects, a Fisher’s z transformation was used because previous studies have shown that a simple arithmetic average of correlations leads to an underestimation of the population mean and hence a biased statistic.9,10 This underestimation occurs because the sampling distribution for correlation coefficients is skewed. To account for this bias, a Fisher Transformation (Eqn. 1) was used.
where z is the Fisher transformed value of each subject’s Pearson correlation coefficient, r.
An arithmetic average was performed on the z values before back-transforming the averaged 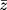 value to give a mean value of
value to give a mean value of 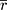 (Eqn. 2),
(Eqn. 2),
Using this back-transformed value of 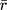 , 95% confidence intervals were calculated using the variance (Eqn. 3) derived from the Taylor Series expansion and Delta method.
, 95% confidence intervals were calculated using the variance (Eqn. 3) derived from the Taylor Series expansion and Delta method.
where k is the number of subjects, 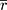 is the Fisher back-transformed mean Pearson correlation coefficient (Eqn. 2), and
is the Fisher back-transformed mean Pearson correlation coefficient (Eqn. 2), and 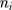 is the number of paired data points (TFI and UFV) for each subject. Note, in order to calculate the variance, a minimum of four pairs of data points were required.
is the number of paired data points (TFI and UFV) for each subject. Note, in order to calculate the variance, a minimum of four pairs of data points were required.
A responder analysis was performed to assess the variability of individual change in TFI by subject and to visualize absolute changes in TFI before and after dialysis by subject. The responder analysis calculated the percent reduction in TFI from baseline to completion of dialysis. A multivariate analysis was performed to determine whether BMI and NYHA class were significantly associated with a percent reduction in TFI before and after dialysis (R Core Team 2018). P values less than 0.05 were determined to be statistically significant.
Results
The mean age (years) of the 20 enrolled subjects was 58. Overall, subjects were mostly men (80%) and Black (65.0%). Subjects had a mean duration of hemodialysis of 7.5 years and 60% of the subjects had a history of heart failure. All demographics are shown in Table 1. The mean body mass index (BMI) of all subjects was 31 with a standard deviation of 9. There were no serious adverse events (SAEs) reported during the study. All AEs were anticipated and related to the dialysis procedure. There were no study device (µCor system) issues related to AEs.
 |
Table 1 Demographic Data of the Subjects Enrolled |
The mean volume of fluid removal was 3.63 L (± 0.88 L). Individual Pearson correlation values of the subject’s TFI to the subject’s UFV are shown in Table 2. Of the 20 subjects, 14 subjects exhibited very strong correlations (r > 0.9), 3 subjects showed a strong correlation (0.7 < r <0.9), and two subjects showed a poor correlation (r < 0.5). Data from subject (202) were not assessed as RF measurements of adequate quality could not be obtained. Figure 2 displays the relationship between TFI and UFV within each subject that had adequate quality measurements. The total number of valid TFI measurements taken for each subject is shown in the third column of Table 2 and ranges from 3 measurements to 35 measurements. One subject’s correlation values (334) were excluded from the Fisher Transform analysis due to the requirement of at least 4 pairs of data for the Fisher Transform formula. For 18 subjects that had at least 4 pairs of data, the average correlation between fluid removal and TFI was r = 0.95 CI [0.92–0.99].
 |
Table 2 Individual Pearson r Correlations of UFV vs TFI with Corresponding Number of Measurements. The Fisher’s Mean r with 95% CI is Reported |
 |
Figure 2 Individual plots by subject of TFI vs UFV (mL) are shown. The number above each plot corresponds to the individual subject study identification number. |
The responder analysis, shown in Figure 3, demonstrated that all subjects experienced a reduction in TFI from baseline to the end of dialysis. The mean reduction of TFI was 5.5% ± 3.8 with a maximum reduction of 13.9%, and minimum reduction of 1.4%. Subject BMI prior to UFV was investigated as a potential influence in the responder analysis, but when subject BMI was compared to the subject’s respective TFI percent change, a weak Pearson correlation value (r) of 0.13 was observed. Of the 11 subjects with TFI data that had an NYHA classification > 0, 6 subjects had a TFI percent change of 0–5%, 4 subjects had a TFI percent change between 5% and 10%, and 1 subject had a TFI percent change greater than 10%. Initial subject BMI and NYHA class were investigated as potential influencers on the TFI reduction. Multivariate regression analysis suggested that reduction in TFI from baseline to the end of dialysis was not significantly associated with neither BMI (p=0.58) nor NYHA (p=0.87).
 |
Figure 3 TFI Responder analysis showing percent change in TFI from beginning of dialysis to the end of dialysis. Some subjects experienced greater absolute TFI changes than other subjects. |
Discussion
There was a strong correlation (0.95 CI [0.92–0.99]) of TFI and UFV when individual Pearson correlation coefficients were averaged through the Fisher’s z transformation. The correlation demonstrated that the µCor system may have utility in tracking total body fluid in dialysis patients.
Some subjects’ TFI responded more strongly to changes in UFV when compared to the TFI response observed in other subjects. We believe these differences in responses could be explained by variables that affect fluid removal in intravascular and interstitial compartments during dialysis. It is possible that for some subjects, even though the fluid is removed from the intravascular compartment, only a small portion of the total fluid is removed from the interstitial compartment during dialysis.11 Supporting evidence of this phenomenon was found in a hemodialysis study, where 31% of subjects following dialysis still had moderate to severe pulmonary congestion demonstrating that fluid removal is often not completely fulfilled.8 It is also possible that individuals with mild pulmonary congestion may experience a more dramatic change in TFI. These possibilities may explain the outcomes of the responder analysis shown in Figure 3 where some subjects exhibited more robust responses in TFI changes than other subjects. All responders however were downward trending.
The findings of the study have implications for the value derived from the patient perspective, both in fluid monitoring during dialysis and fluid monitoring within other disease states such as HF. When patients and clinicians are aware of fluid status, treatments can be tailored to the patient so that benefits are optimized and safety from harmful side effects is reduced. The design of the study device as an RF-based wearable patch also offers advantages compared to current fluid monitors as the device avoids the expertise needed to interpret LUS, and its RF-based measurement is differentiated from traditional bioimpedance monitors where measurements of whole-body fluid status become less sensitive to fluid in the trunk.12,13
Reliable remote monitoring of fluid status provides value to the patient as a safety/efficacy monitor in the home hemodialysis setting, a setting which is the focus of a recent initiative set by the US government to broaden home hemodialysis programs.14 Telemonitoring techniques and devices have recently been identified as innovative approaches for fluid monitoring in dialysis patients, with the potential to improve morbidity and mortality.15 Wearable devices also have the capability to expand upon a singular measurement such as radar or bioimpedance, and integrate multiple biologic signals to provide a comprehensive clinical picture.7 Furthermore, all patients who have access to these data in the setting of home monitoring may become more involved in their care as the consequences of choices that affect the patient’s health may be apparent before symptoms develop.
The study is limited in its scope of 20 hemodialysis subjects. Additionally, all subjects were undergoing chronic hemodialysis and patients new to hemodialysis may have exhibited different findings. The clinical indication for dialysis was unknown for all subjects, and differences in clinical background could have influenced the RF readings. There was a variable number of valid measurements among subjects as only those data points with predetermined signal quality were used for the analysis. The device is limited in its ability to estimate the dry weight of the subject due to the fact that the TFI reading is normalized. Furthermore, total fluid removal measured by UFV may not directly represent changes in the lung fluid captured by a TFI measurement taken in the left lung tissue.
Conclusion
The µCor system provides RF-based measurements of thoracic fluid which correlate well with total body fluid removal in a real-world setting. TFI measurements obtained by the µCor system may be useful in fluid management. Fluid management in dialysis patients serves as a potential model for the management of pulmonary congestion in a variety of clinical settings and conditions characterized by volume overload, including congestive HF. This novel wearable technology has the potential to empower end-stage kidney disease patients and those with HF to improve their clinical outcomes.
Disclosures
MS and RP are employees of ZOLL. JC is a consultant for Achaogen, Akebia, Dynavax, GSK, and Relypsa; research support for Akebia, Astra Zeneca, Cara Therapeutics, Gilead, GSK, ZOLL; and is an employee of DaVita Clinical Research and a stockholder of DaVita Inc. CH is a consultant for Abbvie, Amgen, AstraZeneca, Corvidia, Diamedica, FibroGen, Janssen, NxStage, Pfizer, Relypsa, Sanifit, and University of Oxford; grant/research support for Amgen, Bristol-Myers Squibb, NIDDK/NIH, Relypsa, and University of British Columbia; author royalties from UptoDate; is an employee of Hennepin Healthcare and a stockholder for Boston Scientific, Bristol-Myers Squibb, General Electric, Johnson and Johnson, and Merck. The authors report no other conflicts of interest in this work.
Funding
This trial was funded by ZOLL Medical Corporation.
References
1. Prowle JR, Jirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2013;10:37–47.
2. Payen D, De Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):1–7. doi:10.1186/cc6916
3. Chawla LS, Herzog CA, Costanzo MR, et al. Proposal for a functional classification system of heart failure in patients with end-stage renal disease: proceedings of the Acute Dialysis Quality Initiative (ADQI) XI workgroup. J Am Coll Cardiol. 2014;63(13):1246–1252. doi:10.1016/j.jacc.2014.01.020
4. Rezaeieh SA, Zamani A, Bialkowski KS, Mahmoud A, Abbosh AM. Feasibility of using wideband microwave system for non- invasive detection and monitoring of pulmonary oedema. Nat Publ Gr. 2015;(August):1–11.
5. DeVore AD, Wosick J, Hernandez AF. The future of wearables in heart failure. JACC Hear Fail. 2019;7:11.
6. Efremov SM, Kuzkov VV, Fot EV, et al. Lung ultrasonography and cardiac surgery: a narrative review. J Cardiothorac Vasc Anesth. 2020;34.
7. Reljin N, Posada-Quintero HF, Eaton-Robb C, et al. Machine learning model based on transthoracic bioimpedance and heart rate variability for lung fluid accumulation detection: prospective clinical study. JMIR Med Informatics. 2020;8(8):e18715. doi:10.2196/18715
8. Mallamaci F, Benedetto FA, Tripepi R, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging. 2010;3(6):586–594. doi:10.1016/j.jcmg.2010.02.005
9. Clayton Silver NP, Dunlap W. Averaging correlation coefficients: should Fisher’s z transformation be used? J Appl Psychol. 1987;72(1):146–148. doi:10.1037/0021-9010.72.1.146
10. Corey D, Dunlap W. Averaging correlations: expected values and bias in combined Pearson and Fisher’s z transformation. J Gen Psychol. 1998;125:245–261. doi:10.1080/00221309809595548
11. Chase SC, Fermoyle CC, Wheatley CM, Schaefer JJ, Olson LJ, Johnson BD. The effect of diuresis on extravascular lung water and pulmonary function in acute decompensated heart failure. ESC Hear Fail. 2018;5(2):364–371. doi:10.1002/ehf2.12253
12. Bracco D, Thiébaud D, Chioléro RL, Landry M, Burckhardt P, Schutz Y. Segmental body composition assessed by bioelectrical impedance analysis and DEXA in humans. J Appl Physiol. 1996;81(6):2580–2587. doi:10.1152/jappl.1996.81.6.2580
13. Kotanko P, Levin NW, Zhu F. Current state of bioimpedance technologies in dialysis. Nephrol Dial Transplant. 2008;23(3):808–812. doi:10.1093/ndt/gfm889
14. Executive Order on Advancing American Kidney Health. Available from: https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/.
15. Chait Y, Derk G, Forfang D, et al. Fostering innovation in fluid management. Kidney Heal Initiat. 2019;(July):1–18.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.