Back to Journals » Cancer Management and Research » Volume 11
A Novel Prognostic Scoring Model Based on Albumin and γ-Glutamyltransferase for Hepatocellular Carcinoma Prognosis
Authors Wang L, Li Q, Zhang J , Lu J
Received 22 September 2019
Accepted for publication 15 November 2019
Published 23 December 2019 Volume 2019:11 Pages 10685—10694
DOI https://doi.org/10.2147/CMAR.S232073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Eileen O'Reilly
Liguang Wang,1,* Qun Li,2,* Jie Zhang,2 Jun Lu3
1Department of Oncology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong 250021, People’s Republic of China; 2Department of Infectious Disease, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong 250021, People’s Republic of China; 3Department of Hepatobiliary Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Lu
Department of Hepatobiliary Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong 250021, People’s Republic of China
Email [email protected]
Aim: To investigate the predictive value of albumin (ALB) and γ-glutamyltransferase (GGT) in hepatocellular carcinoma (HCC) patients undergoing curative resection. We sought to establish a new scoring model for predicting the prognosis of HCC patients undergoing curative resection.
Patients and methods: A retrospective analysis was performed in 303 HCC patients who underwent curative resection. Preoperative risk factors for survival were investigated using univariate and multivariate analyses. On the basis of significant factors, a prognostic scoring model was established. The overall survival (OS) and recurrence-free survival (RFS) were compared between different groups.
Results: Multivariate Cox regression showed that preoperative decreased ALB levels and elevated GGT levels were significantly associated with poor OS and RFS. Multivariate analysis showed that ALB level, GGT level, portal vein tumor thrombus, and tumor number were independent prognostic factors for both OS and RFS. Thereafter, we established a preoperative prognostic scoring model combining the four risk factors. The results revealed that higher risk scores might mean worse OS and RFS.
Conclusion: Preoperative ALB and GGT levels are potentially useful biomarkers for predicting the prognostic outcomes in HCC patients undergoing curative resection. Our new prognostic scoring model qualifies as a novel prognostic predictor for HCC patients after curative resection.
Keywords: albumin, γ-glutamyltransferase, hepatocellular carcinoma, prognostic factor
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers and one of the most common causes of cancer-related deaths globally.1 In China, it ranks as the second and third leading cause of cancer-related deaths in men and women, respectively.2 Although there are many treatment options for HCC, liver resection remains the first-line treatment and is reasonably safe and effective for large HCCs and HCCs with microvascular invasion.3,4 Unfortunately, even after surgery, the long-term outcome of HCC patients is still dismal, with an estimated 5-year overall survival (OS) rate of 50%.5
Alpha-fetoprotein (AFP), one of the first protein tumor markers, has been widely used and accepted since its discovery > 60 years ago.6,7 However, it has low sensitivity and specificity in predicting the prognosis of HCC and has no diagnostic value in small HCCs.8 The tumor-node-metastasis (TNM), Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), and Okuda staging systems have been commonly used in stratifying and assessing the prognosis of HCC. However, these staging systems have some limitations. TNM staging does not consider liver function, whereas BCLC staging is difficult to use for distinguishing patients with early or advanced HCC.9 The Okuda staging system does not consider major vascular invasion.10 The CLIP staging system has inadequate discriminative ability.11 Therefore, an accurate model is needed to predict the prognosis of HCC patients after curative resection.
Serum albumin (ALB) and γ-glutamyltransferase (GGT) are two major indices of liver function. ALB is an indicator of nutritional status, whereas GGT is an indicator of the state of liver injury. As a nutrition indicator, ALB can stabilize cell growth and exert antioxidant effects against carcinogens.12 Therefore, low ALB level not only indicates insufficient liver synthesis but also reflects a lack of protection against tumor growth.13 GGT is an enzyme present in the liver, kidney, pancreas, spleen, and other tissues. To date, many clinical studies have reported a high level of GGT in patients with primary or secondary HCC and that elevated GGT levels could predict the prognosis of HCC.14–16 Wu et al17 reported that GGT was a significant prognostic factor and preoperative GGT level could predict the prognosis of patients with hepatitis B virus (HBV)-related HCC treated with curative liver resection.
The purpose of this study was to investigate whether ALB and GGT could be used as prognostic markers for HCC after curative resection over a long-term follow-up period. Moreover, we aimed to introduce a new scoring model based on preoperative serum ALB level, GGT level, portal vein tumor thrombus (PVTT), and tumor number, which are independent predictors in HCC patients, and to investigate the prognostic value of our new scoring model in HCC patients undergoing curative resection.
Patients and Methods
A total of 303 patients who were initially diagnosed with HCC after curative liver resection between October 2010 and July 2015 at the Department of Hepatobiliary Surgery of Shandong Provincial Hospital were enrolled in this study. Patients who met the following criteria were included in this retrospective study: (1) pathologically proven HCC, (2) no other treatments before surgery, (3) no other coexisting solid tumors and hematological diseases, (4) complete laboratory data and follow-up records, and (5) Child-Pugh grade A or B. This study was conducted in accordance with the Declaration of Helsinki. And the study was approved by the Ethics Committee of Shandong Provincial Hospital. All included patients provided signed consent for participation in the study. Written informed consent for the use of clinical data was obtained at the time of surgery. Clinical information was obtained from the medical archives.
Data Collection
For our study cohort, we collected the demographics, preoperative laboratory test results, and tumor-related characteristics, including ALB, GGT, sex, age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TB), Child-Pugh grade, hepatitis B surface antigen, AFP, liver cirrhosis, lymph node metastasis, PVTT, tumor size, tumor number, and TNM stage (according to the 8th American Joint Committee on Cancer staging system). All information was obtained from the hospital database and extensively reviewed. Routine examinations of these 303 HCC patients, including blood examination and imaging evaluations such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI), were performed within 4 days before surgery.
Follow-Up
After curative liver resection, patients were followed up every 3 months in the first 2 years and every 6 months thereafter. During the follow-up, routine physical examination, liver function tests, serum AFP level measurement, and abdominal ultrasound were conducted. When recurrence of HCC was suspected, abdominal enhanced CT scan, MRI, and positron emission tomography-CT were selectively performed. Patients with recurrence were treated with secondary treatments, such as surgery, hepatic artery embolization chemotherapy, and radiofrequency ablation. The primary end points were OS and recurrence-free survival (RFS). OS was calculated from the date of operation to the date of death or the last follow-up. RFS was calculated from the date of operation to the date of first recurrence or the last follow-up (for patients without recurrence).
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation. Categorical data were analyzed using the χ2 test or Fisher’s exact test. The optimal cutoff points of ALB and GGT were measured using X-tile software. Univariate and multivariate Cox proportional hazard regression analyses were performed to determine the independent prognostic factors. Kaplan-Meier survival curves with log rank test were used to compare the differences between different HCC groups. The area under the receiver operating characteristic curve (ROC) curve (AUC) for the scoring model was calculated and compared with other prognostic predictors and other staging systems. A P-value of 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 303 HCC patients who underwent curative resection were enrolled in this study. Of them, 254 were men and 49 were women, with a male-to-female ratio of 5.18:1. The median age was 55 years (range 22–77 years). The median follow-up was 41 months (range 2–69 months). Of the 303 HCC patients, 85.81% (260 subjects) were positive for HBV infection. According to the TNM stage, there were 224 patients with stage I or II, 79 patients with stage III, and no patient with stage IVb HCC. Vascular invasion was found in a minority of patients (n = 45). In our study, there were 31 patients with type I PVTT (tumor thrombus in the segmental branches of the portal vein or above) and 14 patients with type II PVTT (tumor thrombus extending to the right or the left portal vein). The 1-, 3-, and 5-year OS rates in all patients included in our study were 86.8%, 60.7%, and 43.7%, respectively. The baseline clinical characteristics are presented in Table 1.
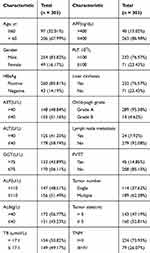 |
Table 1 Clinicopathological Characteristics of HCC Patients, n (%) |
Determination of Cutoff Values
The optimal cutoff values of ALB and GGT, which determined by OS, were established using X-tile software. The results of X-tile analysis revealed that the optimal cutoff points for ALB and GGT levels were 40 g/L and 75 IU/L, respectively. Subsequently, ALB level was stratified into ≤ 40 g/L or > 40 g/L and GGT level was stratified into ≤ 75 IU/L or > 75 IU/L for the subsequent analysis. The optimal cutoff values of ALT, AST, ALP, AFP, platelet count, and TB were used the cut-off value of hospital routine use.
Factors Associated with OS and RFS in HCC Patients
Prognostic factors affecting OS and RFS were analyzed. Univariate analysis revealed that AST, ALB, GGT, ascites, PVTT, tumor number, tumor size, and TNM stage were prognostic factors associated with OS. These factors were assessed using multivariate Cox regression analysis, which showed that ALB, GGT, PVTT, tumor number, and TNM stage could serve as independent predictors of poor OS (Table 2). With respect to RFS, in univariate analysis, AST, ALB, GGT, ascites, PVTT, tumor number, tumor size, and TNM stage were correlated with RFS. All of these eight preoperative factors were entered into multivariate regression analysis. The results showed that ALB, GGT, PVTT, ascites, and tumor number were independent prognostic predictors of poor RFS (Table 3).
 |
Table 2 Prognostic Factors in Univariate and Multivariate Analyses of OS |
 |
Table 3 Prognostic Factors in Univariate and Multivariate Analyses of RFS |
Survival Analysis
ALB was decreased in 131 of 303 patients (43.23%). A decreased preoperative ALB level was significantly associated with poor OS and RFS. The cumulative 1-, 3-, and 5-year OS rates were 98.4%, 75.8%, and 66.7%, respectively, in patients with high ALB level, which were significantly higher than those in patients with low ALB level (94.5%, 52.3%, and 43.7%, respectively) (P < 0.001, Figure 1A). On the other hand, the cumulative 1-, 3-, and 5-year RFS rates were 80.1%, 63.7%, and 54.1%, respectively, in patients with high ALB level, which were significantly higher than those in patients with low ALB level (62.9%, 36.7%, and 30.2%, respectively) (P < 0.001, Figure 1B).
 |
Figure 1 Kaplan–Meier curves for overall (A and C) and recurrence-free survival (B and D) probability according to preoperative albumin and γ-glutamyltransferase respectively. |
GGT was elevated in 133 of 303 patients (43.89%). An elevated preoperative GGT level was significantly associated with inferior OS and RFS. The 1-, 3-, and 5-year OS rates were 94.5%, 78.3%, and 70.1%, respectively, in the low GGT group and 84.2%, 49.7%, and 38.8%, respectively, in the high GGT group (P < 0.001, Figure 1C). The 1-, 3-, and 5-year RFS rates were 83.7%, 62.5%, and 54.3%, respectively, in the low GGT group and 63.2%, 34.4%, and 29.4%, respectively, in the high GGT group (P < 0.001, Figure 1D).
Construction of the Preoperative Prognostic Scoring Model
Inspired by the preoperative prognostic score published by Xu et al18 for HCC patients who underwent curative resection, we established a preoperative prognostic scoring model. In our study, we excluded ascites and TNM stage in the following analysis because the presence of ascites was not an independent prognosis factor of OS and TNM stage was not an independent predictor of RFS. Therefore, we established the prognostic scoring model by including four parameters (ALB, GGT, PVTT, and tumor number) in HCC patients undergoing liver resection. Each factor was given a score of 1 when abnormal, and patients were divided into five categories.
Differences in OS and RFS stratified according to the new preoperative scoring model are shown in Figure 2. The 5-year OS in patients with a score of 0, 1, 2, 3, and 4 was 80.1%, 69.6%, 53.2%, 28.7%, and 0%, respectively (P < 0.001, Figure 2A). With respect to RFS, the 5-year survival for patients with a score of 0, 1, 2, 3, and 4 was 63.9%, 50.2%, 44.6%, 10.1%, and 0%, respectively (P < 0.001, Figure 2B). A shown in the figure, the OS and RFS of patients with a score of 4 sharply decreased compared with those with a score < 4 and patients with a score of 0 had the best survival; however, no significant difference was observed between patients with a score of 3 and 4 in the RFS curve.
 |
Figure 2 Varied outcomes of hepatocellular carcinoma patients as classified by different prognostic scores (A and B). |
Comparison of Predictive Ability Between the New Scoring Model and Other Parameters and Staging System
The predictive value of the preoperative prognostic scoring model compared with ALB, GGT, PVTT, and tumor number was assessed using univariate Cox proportional hazard regression analysis (Table 4). We also included all of these parameters in ROC analysis (Figure 3). The predictive ability was compared using the AUC for OS (Table 5). The AUC for the new scoring model was 0.696 (0.636–0.757), which indicates that the scoring model was the strongest predictor among other prognostic indicators (ALB, GGT, PVTT, and tumor number) of survival in patients with HCC.
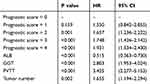 |
Table 4 Univariate Cox Regression Analysis of Score Model and Other Prognostic Parameters |
 |
Table 5 Comparison of Predictive Ability of Score Model and Other Prognostic Parameters |
 |
Figure 3 Predictive ability of the prognostic score was compared with other clinical parameters by ROC curves. |
We also compared our new scoring model with other commonly used staging systems, such as the BCLC, TNM, CLIP, and Okuda staging systems, through ROC curve analysis, and the results are shown in Supplementary Table 1 and Supplementary Figure 1.
Discussion
Our study was mainly focused on the diagnostic roles of ALB and GGT and systematically explored the prognostic roles of these biomarkers. We successfully established a novel and effective score model including ALB, GGT, PVTT, and tumor number, which were all independent prognostic predictors in our study. The Kaplan-Meier survival analysis showed that patients with higher scores had worse outcomes. Our simple and practical model could predict the prognosis of HCC patients and could be used to guide clinical decision making.
Notably, as a nutrition index, ALB reflects the protein synthesis function of the liver and is used for determining the albumin-bilirubin grade, which has been shown to predict long-term survival in HCC patients undergoing surgical resection.19,20 Moreover, the presence of ALB in serum reduces the phosphorylation of Rb proteins, suppresses tumor cell proliferation, and exerts antioxidant effects against carcinogens.21 Recently, ALB has been shown to be not only a predictor of liver cancer but also a popular predictor of survival in many other malignancies, including colorectal cancer and renal cell carcinoma.22,23
With respect to GGT, it has been considered a biomarker of liver disease and alcohol abuse.24 At present, increasing studies are investigating whether GGT plays an important role in predicting prognosis in HCC patients.17,25 The studies have shown that GGT may be associated with worse liver function by inducing DNA instability and subsequent oncogenesis.26 GGT is also associated with inflammation, and some inflammatory factors are products of GGT.27 Moreover, there is increasing evidence indicating that systemic inflammatory response plays an important role in cancer progression.28 Our data showed that ALB and GGT are predictors for patients with HCC after curative hepatectomy over a long-term follow-up, and revealed that preoperative decreased ALB levels and elevated GGT levels were significantly associated with shorter OS and RFS.
It has been demonstrated that recurrence after HCC removal is associated with tumor size, tumor number, major vascular invasion, and liver function status.10 The results of our study are consistent with the above-mentioned results and demonstrated that PVTT and tumor number were independent prognostic predictors of HCC. PVTT is a common complication indicating extremely poor prognosis in HCC patients, and approximately 40% HCC of patients have PVTT at diagnosis.29 For HCC patients with PVTT, the treatments in the East and West are still controversial. In Western countries, the first-line management is nonsurgical treatment, such as molecular targeted therapy, transarterial chemoembolization, or ablation therapy.30 Conversely, in China, an increasing number of studies have suggested that surgical treatments were more beneficial than nonsurgical treatments.31–33 Therefore, multicenter studies with a large sample size are needed for selecting treatment for patients with PVTT.
If a prediction model for HCC prognosis could be developed, it will provide clinicians better treatment recommendations. Thus, from the results of multivariate analysis, we established a simple preoperative prognostic scoring model with an AUC of 0.696, which is superior to the AUC of ALB, GGT, PVTT, and tumor number. Higher risk scores might predict shorter OS and RFS. However, no significant difference was seen between patients with a score of 3 and 4 in the RFS curves, which may be related to our small sample size. It is worth mentioning that our new scoring model is easily available. ALB and GGT are routine preoperative examination items and tumor number and PVTT could be obtained from preoperative imaging examinations.
According to our new scoring model, the 5-year OS rates in patients with a score of 0, 1, 2, 3, and 4 were 80.1%, 69.6%, 53.2%, 28.7%, and 0%, respectively. Combined with the operational risk, complications, and cost of surgery, our new scoring model could help surgeons evaluate the surgical benefits and could provide further guidance for the choice of treatment for HCC patients. The higher the score, the worse the prognosis of the patient, which means that surgery would not be recommended.
However, the current study also has several limitations. First, selection, withdrawal, and other clinical biases were inevitable because our study had a retrospective design. Second, all data were collected from a single medical center and our sample size was small. For these reasons, large-scale, independent, prospective, and multicenter cohort studies are needed to validate the present results.
Conclusion
Preoperative low ALB level and high GGT level were associated with poor prognosis in HCC patients after hepatectomy. Our results confirmed that our new prognostic score qualifies as a novel prognostic predictor in HCC patients after curative resection.
Abbreviations
ALB, Albumin; GGT, γ-glutamyltransferase; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HCC, Hepatocellular carcinoma; TB, total bilirubin; AFP, alpha-fetoprotein; PVTT, Portal vein tumor thrombus.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Song P, Feng X, Zhang K, et al. Screening for and surveillance of high-risk patients with HBV-related chronic liver disease: promoting the early detection of hepatocellular carcinoma in China. Biosci Trends. 2013;7(1):1–6.
3. Wong R, Frenette C. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2011;7(1):16–24.
4. Zhong JH, Rodríguez AC, Ke Y, et al. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion: erratum. Medicine. 2015;94(6):1. doi:10.1097/01.md.0000464211.01415.50
5. Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234(1):71–78. doi:10.1097/00000658-200107000-00011
6. Sauzay C, Petit A, Bourgeois AM, et al. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clinica Chimica Acta. 2016;463:39–44. doi:10.1016/j.cca.2016.10.006
7. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019. doi:10.1111/liv.14223
8. Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56(4):1371–1379. doi:10.1002/hep.v56.4
9. Hsu CY, Hsia CY, Huang YH, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116(12):3006–3014. doi:10.1002/cncr.25044
10. Li Y, Ruan DY, Yi HM, Wang GY, Yang Y, Jiang N. A three-factor preoperative scoring model predicts risk of recurrence after liver resection or transplantation in hepatocellular carcinoma patients with preserved liver function. Hepatobiliary Pancreat Dis Int. 2015;14(5):477–484. doi:10.1016/S1499-3872(15)60412-X
11. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38(3):207–215. doi:10.1007/s005350300038
12. Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. doi:10.1016/j.jhep.2014.04.012
13. Xiong JP, Long JY, Xu WY, et al. Albumin-to-alkaline phosphatase ratio: A novel prognostic index of overall survival in cholangiocarcinoma patients after surgery. World J Gastrointest Oncol. 2019;11(1):39–47. doi:10.4251/wjgo.v11.i1.39
14. Xia J, Song P, Sun Z, Sawakami T, Jia M, Wang Z. Advances of diagnostic and mechanistic studies of gamma-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov Ther. 2016;10(4):181–187. doi:10.5582/ddt.2016.01052
15. Ma H, Zhang L, Tang B, et al. Gamma-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol. 2014;21(9):3084–3089. doi:10.1245/s10434-014-3724-4
16. Shi S, Chen Q, Ye L, et al. Prognostic value of systemic inflammation score in patients with hepatocellular carcinoma after hepatectomy. Oncotarget. 2017;8(45):79366–79375. doi:10.18632/oncotarget.18121
17. Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36(Pt A):143–151. doi:10.1016/j.ijsu.2016.10.033
18. Xu XS, Wan Y, Song SD, et al. Model based on gamma-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World j Gastroenterol. 2014;20(31):10944–10952. doi:10.3748/wjg.v20.i31.10944
19. Toyoda H, Lai PB, O’beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016;114(7):744–750. doi:10.1038/bjc.2016.33
20. Chan AW, Chong CC, Mo FK, et al. Incorporating albumin-bilirubin grade into the cancer of the liver Italian program system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32(1):221–228. doi:10.1111/jgh.2017.32.issue-1
21. Nojiri S, Joh T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int J Mol Sci. 2014;15(3):5163–5174. doi:10.3390/ijms15035163
22. Nazha B, Moussaly E, Zaarour M, Weerasinghe C, Azab B. Hypoalbuminemia in colorectal cancer prognosis: nutritional marker or inflammatory surrogate? World J Gastrointest Surg. 2015;7(12):370–377. doi:10.4240/wjgs.v7.i12.370
23. Chen Z, Shao Y, Fan M, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(11):14893–14900.
24. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355. doi:10.1080/20014091084227
25. Pang Q, Bi JB, Wang ZX, et al. Simple models based on gamma-glutamyl transpeptidase and platelets for predicting survival in hepatitis B-associated hepatocellular carcinoma. Onco Targets Ther. 2016;9:2099–2109. doi:10.2147/OTT.S101465
26. Wang Z, Song P, Xia J, Inagaki Y, Tang W, Kokudo N. Can gamma-glutamyl transferase levels contribute to a better prognosis for patients with hepatocellular carcinoma? Drug Discov Ther. 2014;8(3):134–138. doi:10.5582/ddt.2014.01025
27. Daubeuf S, Accaoui MJ, Pettersen I, Huseby NE, Visvikis A, Galteau MM. Differential regulation of gamma-glutamyltransferase mRNAs in four human tumour cell lines. Biochim Biophys Acta. 2001;1568(1):67–73. doi:10.1016/S0304-4165(01)00201-X
28. Bouman L, Sanceau J, Rouillard D, Bauvois B. gamma-Glutamyl transpeptidase expression in Ewing’s sarcoma cells: up-regulation by interferons. Biochem J. 2002;364(Pt 3):719–724. doi:10.1042/bj20011854
29. Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62–67. doi:10.1002/hep.510290145
30. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England). 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
31. Liang L, Chen TH, Li C, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB. 2018;20(12):1119–1129. doi:10.1016/j.hpb.2018.06.1804
32. Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget. 2017;8(20):33911–33921. doi:10.18632/oncotarget.15411
33. Luo F, Liao R. Hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: benefit or not. Hepatology. 2019. doi:10.1002/hep.30872
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
