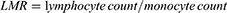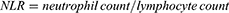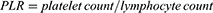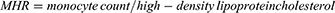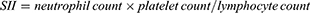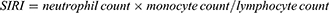Back to Journals » Journal of Inflammation Research » Volume 15
A Novel Nomogram Integrated with Systemic Inflammation Markers and Traditional Prognostic Factors for Adverse Events’ Prediction in Patients with Chronic Heart Failure in the Southwest of China
Authors Liu Z , Zhang R, Xv Y, Wang J, Chen J, Zhou X
Received 24 March 2022
Accepted for publication 18 October 2022
Published 20 December 2022 Volume 2022:15 Pages 6785—6800
DOI https://doi.org/10.2147/JIR.S366903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Zhaojun Liu,1,* Ren Zhang,1,* Yingjie Xv,2 Jinkui Wang,3 Jie Chen,1 Xiaoli Zhou1
1Department of Cardiology, First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Urology, First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Urology; Ministry of Education Key Laboratory of Child Development and Disorders; National Clinical Research Center for Child Health and Disorders (Chongqing); China International Science and Technology Cooperation Base of Child Development and Critical Disorders; Chongqing Key Laboratory of Pediatrics; Children’s Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoli Zhou, Email [email protected]
Objective: Inflammation contributes to the pathogenesis and progression of heart failure (HF). This study aimed to construct a nomogram based on systemic inflammatory markers and traditional prognostic factors to assess the risk of adverse outcomes (cardiovascular readmission and all-cause death) in patients with chronic heart failure (CHF).
Methods: Data were retrospectively collected from patients with HF admitted to the Department of Cardiovascular Medicine at the First Affiliated Hospital of Chongqing Medical University from January 2018 to April 2020, and each patient had complete follow-up information. The follow-up duration was from June 2018 to May 31, 2022. 550 patients were included and randomly assigned to the derivation and validation cohorts with a ratio of 7:3, and prognostic risk factors of CHF were identified by Cox regression analysis. The nomogram chart scoring model was constructed.
Results: The Cox multivariate regression analysis showed that traditional prognostic factors such as age (P=0.011), BMI (P=0.048), NYHA classification (P< 0.001), creatinine (P< 0.001), and systemic inflammatory markers including LMR (P=0.001), and PLR (P=0.015) were independent prognostic factors for CHF patients. Integrated with traditional and inflammatory prognostic factors, a nomogram was established, which yielded a C-index value of 0.739 (95% CI: 0.714– 0.764) in the derivation cohort and 0.713 (95% CI: 0.668– 0.758) in the validation cohort, respectively. The calibration curves exhibited good performance of the nomogram in predicting the adverse outcomes for patients with CHF. In subgroups (HFrEF, HFmrEF, and HFpEF groups), the systematic inflammatory markers-based nomograms proved to be effective prediction tools for patients’ adverse overcomes, as well.
Conclusion: The nomogram combining systemic inflammatory markers and traditional risk factors has satisfactory predictive performance for adverse outcomes (mortality and readmission) in patients with CHF.
Keywords: chronic heart failure, inflammation, prognosis, systemic inflammatory markers, nomogram
Introduction
Chronic heart failure (CHF) is a clinical syndrome characterized by impaired cardiac function, resulting from the stabilization of acute heart failure after an initial episode or from the progressive development of other cardiovascular diseases.1 CHF represents the end stage of various cardiac diseases, and its high rehospitalization rate and mortality impose a substantial public health burden on society.2 Thus, it is essential to assess the prognosis of patients with CHF, as patients at higher risk of poor outcomes could receive more intensive therapy and close monitoring.
Various heart failure (HF) risk stratification models have been previously developed based on different clinical characteristics and biomarkers to tailor the treatment prescription for CHF patients.3–6 However, most of them were mainly designed for the white race, and risk factors for HF vary substantially across world regions due to the racial differences in HF.3 Therefore, the current prediction models maybe not be suitable for evaluating the Chinese CHF population, and a novel prediction model that bears prediction ability for Chinese CHF patients’ prognosis prediction is urgently needed.
Inflammation may contribute to the development of HF after the activation of hemodynamic disturbances, sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS).7–9 It has been reported that inflammation is an important pathophysiological feature of HF and that inflammation markers can be used to assess the severity and the prognosis of patients with HF.10–12 Several systemic inflammatory markers, including lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR) (NLR), monocyte-to-high-density-lipoprotein ratio (MHR), systemic immune inflammation index (SII), and systemic inflammation response index (SIRI) have attracted much attention in recent years. These markers are simple to calculate according to routine blood markers and have the advantage of being inexpensive and easy to detect, which thus have been used in many clinical studies for early assessment of the prognostic risk of various cardiovascular diseases.13–15 This study aimed to develop a novel prognostic nomogram applicable to the southwest Chinese CHF population by combining systemic inflammatory markers and traditional CHF risk factors.
Methods and Materials
Study Population
This was a retrospective study conducted in the First Affiliated Hospital of Chongqing Medical University (Chongqing, China). The study protocol complied with the Declaration of Helsinki and was approved by the hospital’s ethics review board. All procedures included in this study were undertaken as part of routine clinical practice, and the data which could identify subjects were removed. We confirmed that all the data was anonymized and maintained with confidentiality; therefore, the requirement for informed consent has been waived because of the retrospective nature of the current study.
Patients with CHF admitted at the Department of Cardiology in our institution from January 2018 to April 2020 with complete follow-up information were retrospectively included. The inclusion criteria were as follows: (1) age ≥18 years; (2) diagnosed with CHF according to association standards of the”2018 Chinese Heart Failure Diagnosis and Treatment Guidelines” and”2016 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure”;1 (3) New York Heart Association (NYHA) classification II-IV. To minimize the impact of drugs and other diseases on systemic inflammation markers, some patients were excluded from this study. As shown in Figure 1, the exclusion criteria including (1) with acute and chronic infection (n=403); (2) with end-stage liver or renal failure (n=155); (3) with hematological disorders (n=89); (4) with cancer (n=91); (5) with rheumatic immune system diseases (n=103); (6) under glucocorticoids therapy (may affect coagulation) (n=190); (7) with incomplete information (n=45); (8) Patients who were readmitted in other hospitals (n=158). Finally, 550 patients met the inclusive and exclusive criteria and were enrolled in the study cohort, and were randomly divided into derivation and validation sets with a 7: 3 ratios (The derivation set was used to train the model, giving the model inputs and corresponding outputs and letting the model learn the relationship between them, while the validation set was the result of the input data on the final model, the output of the trained model on the simulated ‘new’ input data).
 |
Figure 1 Flowchart of recruiting study cohorts. |
Clinical and Laboratory Data Collection
Demographic characteristics [including age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), body mass index (BMI), and smoking status], preexisting comorbidities (including diabetes mellitus, hypertension, coronary atherosclerotic heart disease, atrial fibrillation, dyslipidemia, stroke, and ventricular assist device) and medical history [including β-blockers, angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), aspirin, statin, diuretics, antiplatelet drugs, oral anticoagulants, calcium channel blockers, and nitrates] were collected from the electronic medical records in our institute.
Blood samples were obtained on admission for laboratory determination, B-type natriuretic peptides (BNP) concentration and blood routine tests (platelet count, neutrophil count, lymphocyte count, and monocyte count) were conducted. Fasting blood samples were collected the following day, and biochemical parameters including blood urea nitrogen (BUN), creatinine, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), Apolipoprotein-A, Apolipoprotein-B, Lipoprotein(α) and high sensitivity C reactive protein (hs-CRP) were measured via an auto-analyzer.
LVEF assessment was based on 2D echocardiography using the quantitative 2D biplane volumetric Simpson method from 4- and 2-chamber views.
The continuous variables such as age (I: <60, II: 60–69, III: 70–79, IV: >79) and BMI (I:<18.5, II:18.5–24.9, III: 25–29.9, IV: >29.9) were transformed into categorical variables according to the criteria of studied documents16,17; SBP, DBP, HR, BNP, LEVF, platelet count, neutrophil count, lymphocyte count, monocyte count, BUN, TG, TC, creatinine, HDL-C, LDL-C, Apolipoprotein-A, Apolipoprotein-B, and Lipoprotein(α) were transformed into categorical variables on basis of their corresponding best cut-off values. The properties of other parameters were unchanged.
Calculation of the Systemic Inflammation-Related Marker
The systemic inflammation-related markers, including LMR, NLR, PLR, MHR, SII, and SIRI were calculated using the following equations:18–21
Study Endpoints
The adverse outcome of this study was all-cause death or cardiovascular readmission at 1 and 3 years after discharge (Cardiovascular readmission is the first unplanned hospital readmission for an HF episode within 1 year after discharge or 3 years after discharge). Starting in June 2018, electronic medical record review was conducted every six months to collect information on a patient’s cardiovascular readmission or all-cause death in the past period. The follow-up terminated the study on May 31, 2022 and the time of hospitalization or death of our study population was recorded.
Establishment and Evaluation of Prediction Nomogram
The included CHF patients were randomly divided into the derivation and validation cohorts in a 7:3 ratio by random sampling, and baseline characteristics of the two groups were compared. Univariate and multivariate Cox analyses were performed to identify potential prognostic factors for CHF. A nomogram was constructed based on statistically significant factors identified by the multivariate analysis. Discrimination and calibration were utilized to evaluate the performance of the nomogram.
To evaluate the ability of the model to predict an adverse outcome, we calculated the calibration of the model using 1000 bootstrap samples to decrease the overfit bias. The consistency index (C-index) and receiver operating characteristic (ROC) curves were used to evaluate the nomogram’s predictive performance and discrimination ability. The clinical effectiveness of the resulting model was evaluated by a decision curve analysis (DCA).
Survival Analysis
The collected clinical data were fed into univariate and multivariate Cox regression analyses to detect the prognostic predictor of patients. Risk scores were calculated for each patient according to the constructed nomogram, and patients were then divided into high-risk and low-risk groups by the median value of the score. Kaplan-Meier curve analysis was conducted to describe the survival time of CHF patients, and the Log-Rank method was used to compare the differences in Kaplan-Meier curves between the two groups.
Statistical Analysis
The skewed distribution of continuous variables was expressed as median (25th percentile, 75th percentile) and was analyzed by the Mann–Whitney U-test. Categorical variables were described in terms of the number of cases (percentages), and baseline characteristics of the derivation and validation cohorts were compared using a chi-square test (for one of the expected frequencies is greater than 5) or Fisher’s exact test (for sample size n < 40 or theoretical frequency T < 1).
All statistical analyses were performed using R software (version 4.0.2) and were statistically significant at P<0.05.
Results
Clinical Characteristics of the Study Cohort
550 patients (male: female=262: 288) with CHF were finally enrolled. Clinical characteristics of the study cohort are listed in Table 1. The median follow-up time was 20.25 months, and the incidence of adverse outcome was 60%.
 |
Table 1 Baseline Characteristics of Subjects |
Systemic inflammatory markers were compared between the adverse outcome group and the group without adverse outcomes (Table 2). As a result, the adverse outcome group presented lower levels of LMR (P < 0.001) and higher levels of NLR (P < 0.001), PLR (P < 0.001), SII (P = 0.001), and SIRI (P < 0.001). No significant difference was found in the MLR level between the two groups.
 |
Table 2 Comparison of Baseline Characteristics of Patients in Different Outcomes |
Baseline Characteristics of Patients in the Derivation and Validation Cohorts
The study population was randomly divided into the derivation (386 patients) and validation cohorts (164 patients) in a 7:3 ratio, and the baseline characteristics of the patients in the two groups were analyzed (Table 3). Except for PLR (p= 0.032), SII (p= 0.020), and SIRI (p= 0.046), no statistical difference was found in the demographic characteristics and clinical markers between the two groups.
 |  |  |
Table 3 Characteristics of Patients in the Derivation and Validation Cohorts |
Variable Selection
Univariate and multivariate Cox regression analyses were performed. Age, BMI, smoking, hypertension, coronary artery disease, ventricular assist device, NYHA classification, β-blockers, ACEI/ARB, lymphocyte count, monocyte count, BUN, creatinine, hs-CRP and systemic inflammatory markers (LMR, PLR, NLR, SII, and SIRI) were proved to associated with CHF patients with adverse outcomes (all P<0.05) in the univariate Cox analysis. These variables were then processed in multivariate Cox regression analysis (step-backward method) to assess their predictive significance for CHF. As a result, traditional factors including age (P=0.011), BMI (P=0.048), NYHA classification (P<0.001), creatinine (P<0.001), and systemic inflammatory markers including LMR (P=0.001), and PLR (P=0.015) were determined to be independent prognostic factors for adverse outcome in patients with CHF (Table 4).
 |
Table 4 Univariate and Multivariate Cox Analyses of Death or Readmission in Patients with Chronic Heart Failure |
Risk Model Development and Validation
Based on the results of the multivariate Cox regression analysis, three systemic inflammatory markers (PLR and LMR) and traditional risk factors (age, BMI, NYHA classification, and creatinine) were utilized to construct a nomogram, which can provide individualize prediction of the adverse outcome risk (Figure 2). In the derivation and validation cohorts, the C‐index for the nomogram of readmission or death risk was 0.739 (95% CI: 0.714–0.764) and 0.713 (95% CI: 0.668–0.758) with bootstrapping, respectively.
As shown in Figure 3, the AUCs for adverse outcomes in the derivation cohort were 0.725 (95% CI: 0.672–0.778) and 0.726 (95% CI: 0.639–0.813) at 1 year and 3 years, and in the validation cohort were 0.712 (95% CI: 0.623–0.801) and 0.731 (95% CI: 0.635–0.827), which indicated that the nomogram had good discrimination ability. The calibration curve also revealed good agreement between the nomogram’s predictions and the actual outcomes (Figure 4).
Clinical Application Value
In order to measure the clinical application value of the constructed nomogram, DCA was performed in the derivation cohort (Figure 5A) and validation cohort (Figure 5B) had good clinical application in predicting adverse outcomes in CHF patients at 1 and 3 years.
Risk Analysis
The prognostic factors were fed into a nomogram, and risk scores were calculated for each patient. Risk stratification was established using the median of the scores, and the study cohort was divided into high-risk and low-risk groups (Figure 6). In the derivation group, the risk of adverse outcomes at 1 and 3 years for patients in the high-risk group were 49.46% and 92.93%, in the low-risk group were 19.31% and 86.14%. Log-Rank method showed that the KM curves of the high-risk and low-risk groups were significantly different in the two cohorts (derivation group, and validation group).
The Prognostic Values of the Systematic Inflammatory Markers-Based Nomograms in HFrEF, HFmrEF, and HFpEF Groups
In the HFrEF group, univariate and multivariate cox analysis yielded four prognostic factors (Table S1) as NYHA classification (P = 0.010), coronary heart disease (P = 0.002), ACEI/ARB (P = 0.003), and LMR (P = 0.004), which were employed to establish the nomogram (Figure S1A). The constructed nomogram achieved AUC values of 0.705 (95% CI: 0.620–0.790) and 0.673 (95% CI: 0.556–0.791) for the 1- and 3-year adverse outcomes prediction (Figure S1B), separately, with better clinical benefits (Figure S1C).
In the HFmrEF group, only NLR (P=0.002) presented significant difference between patients with adverse outcomes and without via multivariate cox analysis. Thus, the differential variables in the univariate cox analysis (Table S2), including PLR (P=0.012), NLR (P=0.001), SII (P<0.001), and SIRI (P=0.015) were utilized to nomogram construction (Figure S2A). The nomogram yielded AUC values of 0.662 (95% CI: 0.557–0.766) and 0.654 (95% CI: 0.512–0.796) for 1- and 3-year adverse outcome prediction (Figure S2B), separately, with relative fair clinical benefits (Figure S2C).
As a result of the univariate and multivariate cox analysis (Table S3), age (P=0.006), NYHA classification (P=0.012), LMR (P=0.007), and PLR (P=0.027) proved to be independent risk factors for patients’ adverse outcome in the HFpEF group, and a nomogram was built, accordingly (Figure S3A). The established nomogram presented AUC values of 0.731 (95% CI: 0.667–0.794), and 0.723 (95% CI: 0.639–0.806) for 1- and 3-year adverse outcomes prediction (Figure S3B), the DCA analysis also illustrated its better clinical benefits (Figure S3C).
Discussion
Integrated with systemic inflammatory markers (PLR and LMR) and traditional prognostic factors (age, BMI, NYHA classification, and creatinine), this study established a novel nomogram to evaluate the risk of cardiovascular readmission or all-cause mortality in patients with CHF. The nomogram yielded satisfactory predictive performance, with C-index values of 0.739 (95% CI: 0.714–0.764) and 0.713 (95% CI: 0.668–0.758) in the derivation and validation cohorts, respectively. The DCA showed that the model has clinical application and can help clinicians early screen high-risk patients with CHF. Besides, the systematic inflammatory markers-based nomograms proved to be effective prediction tools for prognosis of CHF patients with different cardiac functional grades.
CHF is a complex clinical syndrome whose development is often accompanied by comorbidities such as obesity, hypertension, diabetes, and chronic kidney disease.22 These comorbidities may trigger a systemic inflammatory state that leads to inflammation of microvascular endothelial cells and increased production of reactive oxygen species (ROS), and reduced utilization of nitric oxide (NO), resulting in myocardial cell injury.23 In turn, chronic inflammation caused by myocardial injury drives monocyte infiltration into the myocardium and differentiation into pro-inflammatory macrophages (M1), further leading to poor left ventricular remodeling and diastolic disturbances.23 In addition, activation of classical neurohormonal systems (sympathetic nervous system and renin-angiotensin-aldosterone system) triggers a sustained myocardial inflammatory response that affects the structure and function of the heart.9 Thus, inflammation is an important pathophysiological feature of HF that independently predicts the prognosis of patients with HF.10,11,24
Systemic inflammatory markers (PLR and LMR) are novel inflammatory markers that have been used in recent years in many studies for prognostic risk assessment in oncology and cardiovascular disease.25–27 Compared with traditional inflammatory markers such as interleukin 1 (IL-1), interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α), systemic inflammatory markers are easy to detect and can be obtained through routine blood testing, which is convenient for clinical use and promotion. In this study, systemic inflammatory markers (PLR and LMR) were identified as independent risk factors of adverse outcomes in patients with CHF via univariate and multivariate Cox analysis. A previous study showed that high levels of PLR were an independent risk factor for all-cause mortality in patients with acute HF.28 In concordant with the above study, we also found that PLR is associated with adverse outcomes in patients with CHF, which complemented the prognostic value of PLR in CHF. Another study reported that LMR, a systemic inflammatory index, was strongly associated with the risk of death in patients with HF, and we have proved that LMR was independent risk factor for patients with CHF in former research.29,30 In this study, the results of univariate and multivariate Cox regression showed that LMR was a protective factor for poor prognosis in CHF patients, which was in accordance with the previous studies.
Except for systemic inflammatory markers, traditional markers including age, BMI, NYHA classification, and creatinine were also proved to be risk factors for adverse outcomes in patients with CHF in this study, which were consistent with the results in previous studies.31,32 HF is a quintessential geriatric cardiovascular condition, with a prevalence of 6% in people aged 60–79 years and 12% in people aged 80 years and older, and the prevalence is likely to increase in the future with the aging of the population.33 The relationship between obesity and HF is widely recognized.34 Although obesity is known to have adverse effects on both systolic and diastolic cardiac function and epidemiological data show a strong association between obesity and heart failure, many studies have shown that obese patients with HF have a better prognosis.35 In this study, we found that patients with the lowest BMI were associated with a poor prognosis of CHF, which is consistent with the previous report. NYHA classification is common risk factor for CHF and are recognized as the independent risk factors for HF prognosis.36,37
In previous studies, the model constructed based on cardiopulmonary exercise test duration, the Kansas City Cardiomyopathy Questionnaire (KCCQ) and other clinical markers have good predictive performance for adverse outcomes (death or readmission) in heart failure with reduced ejection fraction (HFrEF) patients.38 However, the model is based on the HF population from the United States and is only applicable to HFrEF patients. Our study included patients with HF in all ejection fraction ranges and was had good applicability to Chinese CHF patients. In another study, researchers developed a model for Chinese patients with HF using markers from cardiopulmonary exercise tests and traditional prognostic risk factors, which showed good predictive performance for poor outcomes in patients with CHF.39 Unfortunately, the cardiopulmonary exercise test indexes included in their model are less frequently tested in rural Chinese hospitals and do not benefit CHF patients in remote areas. Compared with their model, the systemic inflammatory indexes included in our model can be obtained from routine tests, and other indexes can be easily assessed, which are more suitable for use in rural Chinese hospitals. In addition, the nomogram constructed in our study possessed better predictive performance (C-index value: 0.739 vs 0.690). Besides, we explored the prognosis predictive values of the systematic inflammatory markers-based nomograms in subgroups (HFrEF, HFmrEF, and HFpEF groups). After processing all variables in the univariate and multivariate cox analysis, each subgroup yielded at least one systematic inflammatory marker as prognostic factors, and the nomograms were established. As a result, the systematic inflammatory markers-based nomograms proved to be effective adverse outcomes’ prediction tools for patients with different cardiac functional grades.
Our research may contribute to the clinical relevance and public implications in the following terms: First of all, the novel prognostic biomarkers (PLR and LMR) we found provide an innovative, accurate, low-cost and convenient reference that could facilitate physician to better predict the adverse outcome of patients with CHF, which will benefit the development of the clinical strategies. Secondly, the systemic inflammatory prognostic factors (PLR and LMR) may not only be a tool to monitor the prognosis of patients with CHF, but also be a potential therapeutic target in that its underlying pathophysiological mechanisms may affect the disease progression of chronic heart failure. Thirdly, by combining systemic inflammatory prognostic factors (PLR and LMR) and traditional risk factors (age, BMI, NYHA classification, and creatinine) we establish four nomograms that assist the clinicians to evaluate the survival situation of patients with different cardiac function grades, contributing to the development of precision medicine.
Our study has the following limitations:1 this study is a single-center retrospective cohort study with a small sample size, which needs to be validated by a large-scale multicenter study in the future;2 the systemic inflammation involved in this study was collected at the time of hospital admission, which lacks analysis of longitudinal changes in inflammatory markers;3 our model lacks external validation and is not compared with other CHF risk prediction models.
In conclusion, this study constructed a risk prediction model for the prognosis of CHF patients based on systemic inflammatory markers (PLR and LMR) and traditional risk factors (age, BMI, NYHA classification, and creatinine). The prediction model was able to predict the risk of adverse outcomes (all-cause death and readmission for heart failure episodes) in CHF patients at 1 and 3 years, which can be used to guide early screening of high-risk patients and provide a reference tool for clinical treatment decisions.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Data Sharing Statement
All the data generated in the present research are available by the corresponding authors after reasonable request.
Acknowledgments
We thank all the research subjects for their participation. All authors fulfil the relevant criteria for authorship, including participation in the design, analysis, interpretation, drafting, revision and approval of the study and manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. Zhaojun Liu and Ren Zhang contributed equally and thus share first authorship.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ponikowski P, Voors A, Anker S, et al. 2016 Esc guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (Esc). Developed with the Special Contribution of the Heart Failure Association (Hfa) of the Esc. Eur J Heart Fail. 2016;18(8):891–975. doi:10.1002/ejhf.592
2. Ponikowski P, Anker S, AlHabib K, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25. doi:10.1002/ehf2.12005
3. Voors A, Ouwerkerk W, Zannad F, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. 2017;19(5):627–634. doi:10.1002/ejhf.785
4. Simpson J, Jhund P, Lund L, et al. Prognostic models derived in paradigm-hf and validated in atmosphere and the Swedish heart failure registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol. 2020;5(4):432–441. doi:10.1001/jamacardio.2019.5850
5. Suter L, Li S, Grady J, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340. doi:10.1007/s11606-014-2862-5
6. Pugliese N, De Biase N, Gargani L, et al. Predicting the transition to and progression of heart failure with preserved ejection fraction: a weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur J Prev Cardiol. 2021;28(15):1650–1661. doi:10.1093/eurjpc/zwaa129
7. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20(1):248–254. doi:10.1016/0735-1097(92)90167-l
8. Kan H, Finkel M. Interactions between cytokines and neurohormonal systems in the failing heart. Heart Fail Rev. 2001;6(2):119–127. doi:10.1023/a:
9. Adamo L, Rocha-Resende C, Prabhu S, Mann D. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–285. doi:10.1038/s41569-019-0315-x
10. Dick S, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1):159–176. doi:10.1161/circresaha.116.308030
11. Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T. Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol. 2021;12:746494. doi:10.3389/fphys.2021.746494
12. Mann D. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116(7):1254–1268. doi:10.1161/circresaha.116.302317
13. Meng Z, Yang J, Wu J, Zheng X, Zhao Y, He Y. Association between the platelet-lymphocyte ratio and short-term mortality in patients with non-st-segment elevation myocardial infarction. Clin Cardiol. 2021;44(7):994–1001. doi:10.1002/clc.23648
14. Benites-Zapata V, Hernandez A, Nagarajan V, Cauthen C, Starling R, Tang W. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115(1):57–61. doi:10.1016/j.amjcard.2014.10.008
15. Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, sii and siri with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi:10.2147/jir.S283835
16. Gao S, Yin G, Xia Q, et al. Development and validation of a nomogram to predict the 180-day readmission risk for chronic heart failure: a multicenter prospective study. Front Cardiovasc Med. 2021;8:731730. doi:10.3389/fcvm.2021.731730
17. Tang Y, Dai F, Razali N, Tagore S, Chern B, Tan K. Sleep quality and bmi in pregnancy- a prospective cohort study. BMC Pregnancy Childbirth. 2022;22(1):72. doi:10.1186/s12884-022-04414-7
18. Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. doi:10.1038/bjc.2013.785
19. Yang Y, Wu C, Hsu P, et al. Systemic Immune-Inflammation Index (Sii) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi:10.1111/eci.13230
20. Hua X, Long Z, Huang X, et al. The preoperative systemic inflammation response index (Siri) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. 2020;44(4):100560. doi:10.1016/j.currproblcancer.2020.100560
21. Yang A, Liu J, Tao W, Li H. The diagnostic and predictive role of Nlr, D-Nlr and Plr in Covid-19 patients. Int Immunopharmacol. 2020;84:106504. doi:10.1016/j.intimp.2020.106504
22. Paulus W, Tschöpe C, Novel A. Paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi:10.1016/j.jacc.2013.02.092
23. Glezeva N, Baugh J. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev. 2014;19(5):681–694. doi:10.1007/s10741-013-9405-8
24. O’Connor C, Starling R, Hernandez A, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi:10.1056/NEJMoa1100171
25. Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative peripheral blood Neutrophil-to-Lymphocyte Ratios (Nlr) and Platelet-to-Lymphocyte Ratio (Plr) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Translat Lung Cancer Res. 2021;10(2):866–877. doi:10.21037/tlcr-20-997
26. Kurtul A, Duran M. The correlation between lymphocyte/monocyte ratio and coronary collateral circulation in stable coronary artery disease patients. Biomark Med. 2017;11(1):43–52. doi:10.2217/bmm-2016-0179
27. Graziano V, Grassadonia A, Iezzi L, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. 2019;44:33–38. doi:10.1016/j.breast.2018.12.014
28. Ye G, Chen Q, Chen X, et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: a cohort study. Sci Rep. 2019;9(1):10639. doi:10.1038/s41598-019-47143-2
29. Silva N, Bettencourt P, Guimarães J. The lymphocyte-to-monocyte ratio: an added value for death prediction in heart failure. Nutr Metabol Cardiovasc Dis. 2015;25(11):1033–1040. doi:10.1016/j.numecd.2015.07.004
30. Liu Z, Xv Y, Liu X, Zhou X. Associations of systemic inflammatory markers with the risks of chronic heart failure: a case-control study. Clinics. 2022;77:100056. doi:10.1016/j.clinsp.2022.100056
31. Sciomer S, Moscucci F, Salvioni E, et al. Role of gender, age and bmi in prognosis of heart failure. Eur J Prev Cardiol. 2020;27:46–51. doi:10.1177/2047487320961980
32. Chicco D, Jurman G. Machine learning can predict survival of patients with heart failure from serum creatinine and ejection fraction alone. BMC Med Inform Decis Mak. 2020;20(1):16. doi:10.1186/s12911-020-1023-5
33. Benjamin E, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi:10.1161/cir.0000000000000659
34. Lavie C, Milani R, Ventura H. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi:10.1016/j.jacc.2008.12.068
35. Horwich T, Fonarow G, Hamilton M, MacLellan W, Woo M, Tillisch J. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795. doi:10.1016/s0735-1097(01)01448-6
36. Kannan A, Janardhanan R. Hypertension as a risk factor for heart failure. Curr Hypertens Rep. 2014;16(7):447. doi:10.1007/s11906-014-0447-7
37. Duarte R, Gonzalez M, Oliveira J, Goulart M, Castro I. Is there an association between the nutritional and functional parameters and congestive heart failure severity? Clin Nutr. 2021;40(5):3354–3359. doi:10.1016/j.clnu.2020.11.008
38. O’Connor C, Whellan D, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the hf-action predictive risk score model. Circ Heart Fail. 2012;5(1):63–71. doi:10.1161/circheartfailure.111.963462
39. Zhuang B, Shen T, Li D, et al. A model for the prediction of mortality and hospitalization in Chinese heart failure patients. Front Cardiovasc Med. 2021;8:761605. doi:10.3389/fcvm.2021.761605
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.