Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
A Novel Multiphase Modified Ketogenic Diet: An Effective and Safe Tool for Weight Loss in Chinese Obese Patients
Authors Wu W, Zhou Q, Yuan P, Qiao D, Deng S, Cheng H, Ren Y
Received 5 March 2022
Accepted for publication 10 August 2022
Published 17 August 2022 Volume 2022:15 Pages 2521—2534
DOI https://doi.org/10.2147/DMSO.S365192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Wenjun Wu,1 Qunyan Zhou,2 Peng Yuan,3 Dan Qiao,2 Shukun Deng,3 Haiyan Cheng,1 Ye Ren1
1Department of Endocrinology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, People’s Republic of China; 2Department of Clinical Nutrition, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, People’s Republic of China; 3Department of Rehabilitation Medicine, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, People’s Republic of China
Correspondence: Wenjun Wu, Department of Endocrinology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, No. 299 Qingyang Road, Wuxi, People’s Republic of China, 214023, Tel +86 510 85351181, Fax +86 510 85737592, Email [email protected] Qunyan Zhou, Department of Clinical Nutrition, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, No. 299 Qingyang Road, Wuxi, People’s Republic of China, 214023, Tel +86 510 85350757, Fax +86 510 85737592, Email [email protected]
Purpose: The aim of the present study was to investigate the effect and safety of a multiphase modified ketogenic diet (MMKD) compared to beinaglutide treatment or lifestyle modification (LM) alone on weight loss in obese patients in China.
Patients and Methods: The present study was conducted in adults with obesity who did not have diabetes with two phases as follows: a 4-week run-in phase to guide diet and exercise, followed by a 12-week intervention phase aiming to lose weight. All participants performed aerobic and resistance exercise, and they were free to select any one of three weight-loss strategies as follows: LM group, 12 weeks of hypocaloric balanced diet (HBD); MMKD group, two cycles of a multiphase diet with each cycle comprised of 2 weeks of ketogenic diet (KD), 2 weeks of transition diet and 2 weeks of HBD; and beinaglutide group, 12 weeks of HBD plus daily injection of beinaglutide (0.4 mg per day). Body weight, body composition and metabolic variables were measured before and after the 12 weeks of treatment.
Results: All intervention strategies had significant weight loss, and the MMKD led to greater weight loss than LM (difference, − 3.7 kg; 95% confidence interval [CI], − 6.1 to − 1.4; P = 0.001) but not beinaglutide (difference, − 1.5 kg; 95% CI, − 4.3 to 1.3; P = 0.587). Waist circumference (WC), fat mass, body fat percentage (BFP) and visceral fat area (VFA) were also significantly decreased, and the MMKD had a greater effect on these parameters than LM or beinaglutide. In addition, significant reductions in blood pressure and homoeostatic model assessment of insulin resistance (HOMA-IR) were observed in all three groups, but the MMKD resulted in the most significant improvement in insulin resistance. Almost no adverse events, except for two cases of dizziness, were observed in the MMKD group, which was significantly fewer events than the other two groups.
Conclusion: These findings demonstrated that the MMKD is an effective and safe treatment for weight loss, thus providing an additional option for obese Chinese patients.
Keywords: dietary therapy, obesity, beinaglutide, lifestyle modification
Introduction
Substantial changes in lifestyle factors such as dietary patterns and sedentary behaviors, have resulted in increases of obesity in China in the past four decades.1 Strong evidence has been established that overweight and obesity increase the risk of major noncommunicable diseases including cardiovascular disease, type 2 diabetes mellitus (T2DM), and cancer, which are associated with premature death and disability.2 Studies have confirmed that at least 5% weight loss is associated with significant clinical benefits for many obesity-related complications.3–5 However, effective lifestyle interventions suitable for Chinese populations are scarce, and there are few approved weight loss medications and low acceptance of bariatric surgery in China.6 Therefore, it is urgent to seek an effective, feasible and easily acceptable approach for weight loss.
One popular diet regimen is the ketogenic diet (KD), a low-carbohydrate and high-fat diet, which has been demonstrated to be effective for weight loss and improvement of metabolic parameters.7,8 Nevertheless, the KD is still not an established strategy for obesity treatment due to low tolerance and some side effects, such as fatigue, dizziness, gastrointestinal complaints, hepatic steatosis, nephrolithiasis, lipid abnormalities, and vitamin deficiency.9 Although the KD under strict medical supervision increases patients’ adherence and reduces the most common side effects, it is still a challenging approach for patients, especially with a high-carbohydrate diet.10 Additionally, the KD is associated with a reduction in consumption of healthy foods, such as whole grains, legumes and vegetables, and the KD is completely different from traditional Chinese food, resulting in an obstacle for implementation. Thus, we designed a multiphase modified ketogenic diet (MMKD) while considering Chinese food habits. The ketogenic phase of the MMKD limits carbohydrate intake to 27% of energy, and the MMKD contains approximately 50 g/day of digestible carbohydrates. Moreover, the MMKD emphasizes the choice of foods rich in whole grains with a low glycemic load, green leafy vegetables, fish and olive oil.
Previous studies have indicated that glucagon-like peptide-1 receptor agonists (GLP-1 RAs) significantly reduce body weight in obese patients by slowing gastric emptying and inhibiting appetite.11–13 Beinaglutide is a recombinant human GLP-1 RA with a 100% protein sequence identity to human GLP-1 (7–36), and it has been approved by the China Food and Drug Administration for the treatment of T2DM. Similar to other GLP-1RAs, the pharmacological efficacy of beinaglutide is not limited to glucose lowering. Significant weight loss (−10.05 kg) is observed in T2DM patients after 3 months of treatment with beinaglutide according to the real-world data.14
To our knowledge, no studies have evaluated the efficacy of KD and GLP-1RAs for weight loss simultaneously. Thus, we designed a head-to-head clinical study to compare the efficacy of MMKD, beinaglutide or lifestyle modification (LM) on weight loss in obese patients in China. The present study included a 4-week run-in phase to guide diet and exercise followed by a 12-week intervention phase aiming to lose weight. All participants performed aerobic and resistance exercise, and they were free to select any one of three weight-loss strategies as follows: LM group, hypocaloric balanced diet (HBD) for 12 weeks; MMKD group, two cycles of a multiphase diet with each cycle comprised of 2 weeks of KD, 2 weeks of transition diet and 2 weeks of HBD; and beinaglutide group, 12 weeks of HBD plus daily injection of beinaglutide (0.4 mg per day). The primary purpose of the present study was to investigate the change in body weight after a 12-week intervention. Changes in body composition parameters, glycemic profiles and lipid profiles were also observed, and all adverse events were recorded.
Materials and Methods
Study Design
Patients attending the weight-loss clinic of Nanjing Medical University affiliated Wuxi People’s Hospital were enrolled in an open label prospective weight-loss intervention study. The study was conducted from June 2018 to February 2020 with two phases as follows: a 4-week run-in phase to instruct all participants in LM followed by a 12-week intervention phase aiming to lose weight with three possible weight-loss programs. The basic design of the study is shown in Figure 1.
 |
Figure 1 Study design. Abbreviations: LM, lifestyle modification; MMKD, multiphase modified ketogenic diet; T, titration of beinaglutide within 2 weeks. |
The trial was approved by the Hospital Ethics Committee (KYLLKS 201806) and registered in the Chinese Clinical Trial Registry (ChiCTR 1800015923) prior to initiation of recruitment. All participants provided written informed consent. This study was performed in compliance with the Declaration of Helsinki.
The primary endpoint was the change in body weight after the 12-week intervention phase. The secondary endpoints included changes in body composition, blood pressure, plasma glucose, glycated hemoglobin 1c (HBA1c), lipid profile, and homoeostatic model assessment of insulin resistance (HOMA-IR) after the 12-week intervention.
Participants
The inclusion criteria were as follows: aged 18 to 60 years; body mass index (BMI) ≥ 30.0 kg/m2 or ≥ 28.0 kg/m2 with one or more comorbidities (hypertension, dyslipidemia, sleep apnea or impaired glucose tolerance); and stable body weight in the previous 3 months. A BMI cutoff of 28.0 kg/m2 was recommended to define obesity by the Working Group on Obesity in China.15 The major exclusion criteria were diabetes and secondary obesity caused by diseases or drugs. The complete list of the exclusion criteria is shown in the Supplementary Appendix (Table S1).
Interventions
During the run-in phase, all participants consumed a HBD with the total calories calibrated by basal metabolic rate multiplied by 1.2. The basal metabolism was measured by bioelectrical impedance analysis (InBody® S10 Medical Body Composition Analyzer, Biospace Co., Ltd., Korea). The macronutrient composition of the HBD was 39%, 37% and 24% of total energy from carbohydrates, fat and protein, respectively.
During the intervention phase, all participants were given the option to select any one of three possible weight-loss programs, namely, LM, MMKD or beinaglutide injection. Patients in the LM arm underwent a HBD for a 12-week intervention period. Patients in the MMKD arm underwent two cycles of a multiphase diet, and each cycle was comprised of 2 weeks of KD intervention with 27% carbohydrates; 2 weeks of transition diet intervention with 29% carbohydrates; and 2 weeks of HBD with 39% carbohydrates. The total calories for the KD were the actual basal metabolism measured by InBody S10. If the basal metabolism exceeded 1500 kcal, it was calculated as 1500 kcal. Regarding the total energy, 27%, 41% and 32% from carbohydrates, fat and protein, respectively. Carbohydrates with a glycemic index less than 55 such as whole grains or bran were selected as the staple food. The total calories, nutrient components and food choices of the diets at each stage of the MMKD have been described in our previous study.16 In the beinaglutide arm, patients consumed a HBD plus received injections of beinaglutide (0.4 mg per day), which was given twice daily as subcutaneous injections and titrated, starting at a dose of 0.1mg per injection and increasing to 0.2 mg per injection within 2 weeks to reduce side effects. Beinaglutide and injector pens were supplied by Shanghai Benemae Pharmaceutical Corporation.
Each participant also performed aerobic exercise designed to meet the World Health Organization recommendations17 during the entire study. Aerobic exercise, namely, brisk walking at a speed of 4.8 km/h, was performed 30 min per day. Each particpant was instructed to gradually increase the walking speed to the target speed of 4.8 km/h in the first 2 weeks in the run-in phase and then maintain this speed for the following study. Resistance exercise was added in the intervention period with 10 sets per day of hip bridges, plank supports, static squatting against the wall, and 60- degree double straight-leg elevation. Each movement was maintained for 30s with a 1 min rest every 5 min. As long as the sufficient exercise volume (duration × intensity) was reached, participants could reduce exercise frequency to 5 times per week with prolonged duration or increased intensity.
Schedule of Visits
After the initial screening visit, selected participants were scheduled for 5 face-to-face visits every 4 weeks ± 3 days and telephone interviews weekly throughout the study (Figure 1). A complete physical, anthropometric and biochemical assessment was performed at visits 1, 3, and 6. A dietary plan and an exercise guide were provided at visit 2. The remaining in-person visits and telephone interviews were to control adherence and evaluation of potential side effects.
All dietary regimens during the study period were conducted by a registered nutritionist and a nutrition technician with an occupational qualification certificate. The nutritionist designed and instructed the diet plan for each participant, and the nutrition technician assisted in the follow-up to ensure effective implementation. Eating patterns and adherence were evaluated from 3 days per visit of dietary records (2 weekdays and 1 weekend day). In the MMKD arm, a WeChat group was established for each participant to monitor morning- urine ketones and program performance as described in our previous study.
The aerobic and resistance exercises were planned and monitored by two rehabilitation physicians who had an exercise prescription specialist certificate. Physical activity level and compliance were evaluated from physical activity records at each visit.
Outcome Measurements
Body weight and height were measured in light clothing without shoes on a calibrated scale (HNH-318, Omron, Japan). Weight loss percentage was calculated using the following equation: weight loss percentage = (baseline body weight (kg) - treatment body weight (kg))/baseline body weight (kg). BMI was calculated using the following formula: BMI = weight (kg)/height squared (m2). Waist circumference (WC) at the middle point between the costal arch and the iliac crest as well as hip circumference (HC) at the symphysis greater trochanter level were measured to the nearest 0.1 cm using a standard flexible nonelastic metric tape. Waist-to-hip ratio (WHR) was calculated using the following formula: WHR = WC (cm)/HC (cm). Sitting systolic and diastolic blood pressures were measured twice using a mercury-gravity manometer after 15 min of rest. All measurements were performed by well-trained nurses
Fat mass, muscle mass, skeletal muscle mass (SMM), body fat percentage (BFP), and visceral fat area (VFA) along with other body composition variables were determined using bioelectrical impedance analysis (InBody® S10).
Total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), triacylglycerol (TG), uric acid, blood urea nitrogen, creatinine, total bilirubin, direct bilirubin, alanine transaminase, aspartate transaminase, alkaline phosphatase, gamma-glutamyl transferase and glucose were measured by photometric assays (Chemistry Immuno-analyzer AU5800, Beckman Coulter, USA). HbA1c was measured by a high pressure liquid chromatography method (VARIANT II Haemoglobin Testing System, BIORAD, USA). Serum insulin concentration was measured by an electrochemiluminescence immunoassay method (Roche Diagnostic Systems). Postprandial levels of glucose (P2hBG) and insulin (P2hINS) were obtained after a standard glucose tolerance test. HOMA-IR was calculated as fasting blood glucose (FBG, mmol/L) multiplied by fasting plasma insulin (FINS, mU/L) and then divided by a constant 22.5.
Statistical Analysis
Intention to treat analysis (ITT) and per-protocol analysis (PP) were performed to determine the primary outcome. PP analysis was performed to determine the secondary outcomes. Subjects with one visit after intervention were included in the ITT analysis, and those who completed the entire study were included in the PP analysis. The carry-forward method was applied for drop-outs in the complete ITT sample.
Categorical variables are presented as numbers (percentages) and were analyzed by Chi-squared test or Fisher’s exact test. Continuous variables with normal distribution are presented as the mean ± standard deviation. Data on insulin and HOMA-IR were ln-transformed prior to statistical analysis. Baseline data among the three arms were analyzed by one-way analysis of variance (ANOVA), and pairwise post-hoc comparisons were analyzed by honest significant difference test. Weight change over time was analyzed by a mixed effects model. The differences between baseline and post-intervention were compared by paired t-test within three separate arms. Changes of outcome variables with the intervention among the three arms were analyzed by covariance analysis.
All statistical analyses were performed in IBM SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA), and graphs were created in GraphPad Prism version 8.3.0 (GraphPad Software, San Diego, CA, USA). P-value < 0.05 was considered statistically significant.
Results
Participant Characteristics
Of the 117 patients screened in the weight-loss clinic, 113 met the participation criteria (2 patients failed due to T2DM, 1 patient failed due to lung cancer and 1 patient failed due to severe thrombocytopenia) and were enrolled in the run-in phase. Moreover, 9 patients dropped out during the run-in phase, resulting in a total of 104 patients (58.7% male and 41.3% female) who were grouped into three study arms with the following completion numbers: 50 of 57 subjects in the LM arm completed the study; 19 of 22 subjects in the MMKD arm completed the study; and 21 of 25 subjects in the beinaglutide arm completed the study (Figure 2).
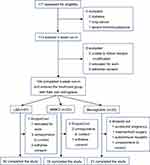 |
Figure 2 Flow-chart of participant enrollment process. Abbreviations: LM, lifestyle modification; MMKD, multiphase modified ketogenic diet. |
The characteristics of the participants before and after the run-in phase are shown in Table S2. During the run-in phase, the participants’ body weight decreased by a mean of 3.3 kg (95% confidence interval [CI], 2.9 to 3.8). This decrease was accompanied by decreases in the WC, HC, body composition, blood pressure, resting heart rate, blood glucose, HbA1c, lipid levels, insulin and HOMA-IR.
The baseline characteristics of the participants in the three study arms are shown in Table 1. There was a significant (P = 0.005) difference in the allocations of females and males among the three study arms as follows (ratio of females to males): 29.8% to 70.2% in the LM arm; 40.9% to 50.1% in the MMKD arm; and 68.0% to 32.0% in the beinaglutide arm. These differences accounted for the baseline differences observed in body fat, fasting insulin level and HOMA-IR.
 |
Table 1 Baseline Characteristics of the Study Participants After the 4-Week Run-in |
Changes in Body Weight and Composition
After 12 weeks of intervention, body weight, BMI, WC, HC, WHR, fat mass, BFP and VFA significantly decreased within the three separate arms (Table S3). Muscle mass, SMM and water significantly decreased in the MMKD and beinaglutide arms but not in the LM arm (Table S3). Treatment effects for these variables had significant differences among the three study arms with the exception of WHR and SMM (Figure 3, Table S3).
Body weight showed a significant decrease over time within each arm (F for time = 109.011, P < 0.001); however, the trend of weight loss was similar across the three study arms (F for treatment = 0.044, P = 0.957) (Figure 3A). From week 8 to 12, weight loss in the beinaglutide arm continued to significantly decrease but not in the other two arms (Figure 3A). After 12 weeks of intervention, the participants’ body weight decreased by a mean of −3.2 kg in the LM arm, −7.0 kg in the MMKD arm and −5.7 kg in the beinaglutide arm. In the MMKD arm, the treatment effect was −3.7 kg (95% CI, −6.1 to −1.4; P = 0.001) compared to LM and −1.5kg (95% CI, −4.3 to 1.3; P = 0.587) compared to beinaglutide (Figure 3B). In the beinaglutide arm, the treatment effect was −2.3kg (95% CI, −4.6 to 0.1; P = 0.058) compared to LM (Figure 3B). The ITT analysis results are shown in Figure S1, and they were consistent with the PP analysis results.
The change in the SMM showed no difference among the three study arms (Figure 3C). The reduction in the fat mass was greater in the MMKD arm than in the LM arm, with a treatment effect of −2.9 kg (95% CI, −5.1 to −0.7; P = 0.005), and this reduction was not observed between the MMKD arm and the beinaglutide arm (P = 0.053) or between the LM arm and the beinaglutide arm (P = 1.000) (Figure 3D). The reduction in the BFP was greater in the MMKD arm than the other two arms, with a treatment effect of −2.1% (95% CI, −4.0 to −0.3; P = 0.020) compared to LM and −2.4% (95% CI, −4.6 to −0.2; P = 0.030) compared to beinaglutide (Figure 3E). Similarly, the reduction in VFA was greater in the MMKD arm than the other two arms, with a treatment effect of −17.1 cm2 (95% CI, −28.8 to −5.5; P = 0.002) compared to LM and −15.8 cm2 (95% CI, −29.7 to −2.0; P = 0.020) compared to beinaglutide (Figure 3F). The reductions of BFP and VFA were not different between the LM arm and the beinaglutide arm (P = 1.000 for both).
The percentages of participants in each arm who had a total weight loss of at least 5% and at least 10% of the baseline body weight after the run-in phase are shown in Figure 3G. The mean weight loss percentage during the 12-week intervention was 8.0% in the MMKD arm, 6.0% in the beinaglutide arm and 3.8% in the LM arm (Figure 3H).
Improvements in Metabolic Indicators
The values for metabolic variables before and after 12 weeks of intervention in all three arms are presented in Table 2. Systolic blood pressure decreased significantly in all three arms with no difference among them, and a similar diastolic blood pressure decrease was observed in the LM and MMKD arms. The resting heart rate displayed no intragroup or intergroup change.
 |
Table 2 Treatment Effects for Metabolic Variables Before and After the 12 Weeks of Intervention |
Regarding lipid metabolism, TG was decreased in the MMKD and beinaglutide arms with a significantly larger decrease in the MMKD arm than in the LM arm (P = 0.008), while HDL-C was significantly increased in the LM arm with a significantly larger increment compared to the beinaglutide arm (P = 0.046). Moreover, LDL-C was increased in the beinaglutide arm with a significantly larger increment than in the LM arm (P = 0.033).
Concerning glucose metabolism, FBG and P2hBG were decreased in all three arms, but only the reduction of FBG in the LM arm reached statistical significance. HBA1c was decreased in the MMKD and beinaglutide arms with a significant reduction in the beinaglutide arm. FINS was significantly decreased in the MMKD and beinaglutide arms, and a similar change was observed for P2hINS in the beinaglutide arm. There were no intergroup differences for changes of FBG, P2hBG, FINS, and P2hINS. Moreover, a predominance change of HOMA-IR was discovered in all three arms with significantly larger reductions with MMKD than with beinaglutide (P = 0.045).
Side Effects
The complete list of all adverse events is provided in Table 3. Adverse events were reported by 29.8% of participants. Two serious adverse events occurred in the beinaglutide group and one in the LM group. In the beinaglutide group, the most common adverse event was nausea (64.0%) followed by dizziness (24.0%), joint injury (20.0%), vomiting (16.0%), upper respiratory tract infection (16.0%) and hypoglycemic symptoms (12.0%). Drug-related adverse events, including gastrointestinal symptoms, hypoglycemic symptoms, fatigue and dizziness, were usually relieved within 6 weeks of beinaglutide treatment. Participants in the other two groups reported fewer adverse events than those in the beinaglutide group. All participants who completed the study felt better than before the study began.
 |
Table 3 Adverse Events During the Study |
Discussion
In the present study, the MMKD led to greater weight loss than LM after 12 weeks of intervention, but the weight loss due to the MMKD was not statistically different compared to that due to beinaglutide. WC, fat mass, BFP and VFA were also significantly decreased among the three groups, and the MMKD had a greater effect on these parameters compared to LM and beinaglutide. Additional health benefits, such as significant improvements in blood pressure and HOMA-IR, were observed with the most benefit in the MMKD group. Importantly, there were fewer reported adverse events in the MMKD group than in the other two groups.
The reduction in body weight was an important achievement of the MMKD. Participants in the MMKD group, beinaglutide group and LM group lost 8.0% (−7.0 kg), 6.0% (−5.7 kg) and 3.8% (−3.2 kg) of baseline weight, respectively. Overall, 84%, 62% and 40% of the participants in the MMKD group, beinaglutide group and LM group lost 5% of weight, and 37%, 10% and 6% of these participants lost 10% of weight, respectively. Notably, our findings showed that the effect the MMKD on weight loss was superior to beinaglutide. The weight loss effect of beinaglutide in this study was similar to that of liraglutide with short-term treatment,18,19 which has been approved by the US Food and Drug Administration and European Medicines Agency for weight management. However, due to a variation in KD regimens and study timelines, the total weight reduction resulting from the MMKD was slightly different from several previous studies in overweight/obese Chinese adults. A non-energy-restricted low-carbohydrate diet with an approximate 50 g/day carbohydrate intake with exercise has been reported to result in more than −2.5 kg of weight loss after 4 weeks of intervention20,21 and −5.27 kg of weight loss after 12 weeks of intervention,22 while an 8-week very low carbohydrate diet with energy restricted to less than 800 kcal/day and carbohydrate intake less than 20 g/day has been reported to cause a weight loss of −8.7 kg.23 Thus, a very low carbohydrate intake combined with very low calorie intake contributes to optimal weight loss. However, this ketogenic regimen requires strict medical supervision and is not suitable for promotion in public practice. The modified KD in the present study limited energy intake to less than 1500 kcal/day and digestible carbohydrate intake to less than 50 g/day by ingesting whole grain products and vegetables, which is a more practical diet.
Significant reductions of WC, fat mass, BFP and VFA were additional important advantages of the MMKD. Moreover, only an average of 0.6 kg of SMM was lost, which agreed with previous studies,24–26 showing that a very-low-calorie KD combined with exercise causes a profound reduction in fat mass with preservation of muscle mass. The restriction of carbohydrates promotes the body to burn fats rather than carbohydrates to provide energy.27 Thus, current evidence suggests that 8–12 weeks of KD combined with resistance training favors fat mass reduction in healthy and trained individuals. Nonetheless, a KD might impair muscle mass accretion induced by resistance training.28 Of note, reintroducing carbohydrates and higher- protein diets (>25% of energy from protein) can help preserve muscle mass.29,30 Fortunately, these factors were taken into account in the design and implementation of the MMKD.
The MMKD improved blood pressure and HOMA-IR index, which was in line with previous studies.7,31 The improvement in blood pressure and insulin resistance through the diet was largely mediated by the reduction in body weight, WC, and fat mass. Although the differences were not significant in the present study, TG, FBG and HBA1c had a decreasing tendency, and HDL-C had an increasing tendency, which agreed with numerous studies.32 In addition, there was no change in TC or LDL-C with the MMKD. However, more detailed lipid subfraction tests are required to determine the cardiovascular benefits of the MMKD.
No clinical side effects were observed in subjects in the MMKD group, except for two cases of mild dizziness. After MMKD treatment, the liver parameters tended to improve, and the renal parameters and uric acid remained unchanged (Table S4). These results indicated a good safety profile of the diet strategy, and it was superior to the reported safety characteristics of the very low-calorie KD. The safety profile of beinaglutide was consistent with previous reports of GLP-1 RAs,33 nausea was common but mostly transient, and it did not affect the compliance of the participants. Unexpectedly, the reported adverse events were more in the LM group than those in the MMKD group. Upper respiratory tract infection and joint injury were the major reported adverse events in the LM group, which may be related to over-implementation.
Lifestyle intervention and pharmacotherapy represent the most effective noninvasive weight loss approaches for the majority of obese patients.34,35 Our data showed all three interventions effectively decreased body weight and were accompanied by improvements in metabolic variables. However, there were differences in the efficacy of weight loss with the MMKD being the most efficacious followed by beinaglutide and LM. Because patients were more likely to select a LM from our data, it is a challenge to select an appropriate method for obese patients to maximize benefits without risk. Based on considerations of efficacy, adverse effects, contraindications and cost for different weight loss interventions, the MMKD may the most suitable approach. For patients who have difficulty in controlling food cravings or lack of satiety, beinaglutide may be helpful in following a diet plan.
The major strength of the present study was the development of a novel modified KD, which is a more relaxed diet plan based on the dietary pattern of a particular ethnic group. The present study demonstrated that this diet results in effective weight loss with low risks and ease of participation as outpatients. Furthermore, the extent of weight loss with this diet was determined by a head-to-head comparison with beinaglutide and LM. Another relevant strength of this study was the tight control of adherence by a multidisciplinary method and monitoring urinary ketones.
Several limitations existed in this study. First, a single-center source of patients and non-randomized design may have introduced some selection bias and baseline difference. Second, the small sample size and the short follow-up prevented the detection of a significant difference in some variables, particularly the secondary endpoints. Third, free selection of treatment options reflected a difference in patient preference and motivation, which influenced the study results. However, the present study indirectly provided real-world evidence. Finally, we should warrant caution when generalizing our results to other populations such as non-Asian individuals, older individuals (>60 years of age), and individuals limited in exercise. Considering all of these limitations, a high quality randomized controlled study with larger cohorts should be performed to validate the present results at short and long terms.
Conclusion
Under the same aerobic and resistance exercise program, patients consuming a MMKD achieved superior weight loss with significant improvements in body composition parameters and fewer side effects compared to beinaglutide and LM. These results indicated that the MMKD is an effective and safe tool suitable for Chinese obese patients to lose weight.
Abbreviations
KD, ketogenic diet; MMKD, multiphase modified ketogenic diet; GLP-1 RAs, glucagon-like peptide 1 receptor agonists; LM, lifestyle modification; T2DM, type 2 diabetes mellitus; HBA1c, glycated hemoglobin 1c; HOMA-IR, homoeostatic model assessment of insulin resistance; BMI, body mass index; HBD, hypocaloric balanced diet; WC, waist circumference; HC, hip circumference; WHR, waist-to-hip ratio; SMM, skeletal muscle mass; BFP, body fat percentage; VFA, visceral fat area; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TG, triacylglycerol; FBG, fasting blood glucose; FINS, fasting plasma insulin; P2hBG, 2h postprandial glucose; P2hINS, 2h postprandial insulin; ITT, intention to treat analysis; PP, per-protocol analysis; CI, confidence interval.
Data Sharing Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy reasons.
Ethics Approval and Informed Consent
The present study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (KYLLKS 201806, 26 April 2018). This trial was registered in the Chinese Clinical Trial Registry (ChiCTR 1800015923). Informed consent was obtained from all subjects involved in the study.
Consent for Publication
All authors gave final approval of the version to be published and agreed to be listed as authors.
Acknowledgments
We thank all the participants in this study. We thank Shanghai Benemae Pharmaceutical Corporation for providing beinaglutide and injector pens. We also thank the expert support from Dalong Zhu and Yan Bi.
Funding
This work was supported by the Project of Jiangsu Health Commission (LGY2019018), the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (BJ2020005) and the Wuxi Science and Technology Development Fund (Y20212024).
Disclosure
The authors declare no conflict of interest in this work.
References
1. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
2. Hu G. More vigorous efforts are needed to fight obesity, a serious public health problem in China. Obesity. 2021;29(10):1580–1581. doi:10.1002/oby.23259
3. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15(3):169–182. doi:10.1111/obr.12113
4. Diabetes Prevention Program Research G. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. doi:10.1016/S2213-8587(15)00291-0
5. Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi:10.1136/bmj.j4849
6. Zeng Q, Li N, Pan XF, Chen L, Pan A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):393–405. doi:10.1016/S2213-8587(21)00047-4
7. Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):5–16. doi:10.1007/s11154-019-09514-y
8. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12(7):2005. doi:10.3390/nu12072005
9. Batch JT, Lamsal SP, Adkins M, Sultan S, Ramirez MN. Advantages and disadvantages of the ketogenic diet: a review article. Cureus. 2020;12(8):e9639. doi:10.7759/cureus.9639
10. Seo JH, Kim HD. Cultural challenges in using the ketogenic diet in Asian countries. Epilepsia. 2008;49:50–52. doi:10.1111/j.1528-1167.2008.01834.x
11. Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi:10.1136/bmj.d7771
12. Ryan D, Acosta A. GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity. 2015;23(6):1119–1129. doi:10.1002/oby.21107
13. Ard J, Fitch A, Fruh S, Herman L. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821–2839. doi:10.1007/s12325-021-01710-0
14. Zhang YL, Zhou C, Li XF, et al. Beinaglutide showed significant weight-loss benefit and effective glycaemic control for the treatment of type 2 diabetes in a real-world setting: a 3-month, multicentre, observational, retrospective, open-label study. Obes Sci Pract. 2019;5(4):366–375. doi:10.1002/osp4.342
15. Zhou BF; Cooperative Meta-Analysis Group of the Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
16. Yuan W, Lu W, Wang H, et al. A multiphase dietetic protocol incorporating an improved ketogenic diet enhances weight loss and alters the gut microbiome of obese people. Int J Food Sci Nutr. 2021;73:1–13.
17. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi:10.1136/bjsports-2020-102955
18. Kadouh H, Chedid V, Halawi H, et al. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J Clin Endocrinol Metab. 2020;105(5):1552–1563. doi:10.1210/clinem/dgz140
19. Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616. doi:10.1016/S0140-6736(09)61375-1
20. Kong Z, Sun S, Shi Q, Zhang H, Tong TK, Nie J. Short-term ketogenic diet improves abdominal obesity in overweight/obese Chinese young females. Front Physiol. 2020;11:856. doi:10.3389/fphys.2020.00856
21. Sun S, Kong Z, Shi Q, et al. Non-energy-restricted low-carbohydrate diet combined with exercise intervention improved cardiometabolic health in overweight Chinese females. Nutrients. 2019;11(12):3051. doi:10.3390/nu11123051
22. Liu X, Zhang G, Ye X, et al. Effects of a low-carbohydrate diet on weight loss and cardiometabolic profile in Chinese women: a randomised controlled feeding trial. Br J Nutr. 2013;110(8):1444–1453. doi:10.1017/S0007114513000640
23. Gu Y, Yu H, Li Y, et al. Beneficial effects of an 8-week, very low carbohydrate diet intervention on obese subjects. Evid Based Complement Alternat Med. 2013;2013:760804. doi:10.1155/2013/760804
24. Lee HS, Lee J. Effects of combined exercise and low carbohydrate ketogenic diet interventions on waist circumference and triglycerides in overweight and obese individuals: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(2):828.
25. D’Abbondanza M, Ministrini S, Pucci G, et al. Very low-carbohydrate ketogenic diet for the treatment of severe obesity and associated non-alcoholic fatty liver disease: the role of sex differences. Nutrients. 2020;12(9):2748. doi:10.3390/nu12092748
26. Gomez-Arbelaez D, Bellido D, Castro AI, et al. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. J Clin Endocrinol Metab. 2017;102(2):488–498. doi:10.1210/jc.2016-2385
27. Barber TM, Hanson P, Kabisch S, Pfeiffer AFH, Weickert MO. The low-carbohydrate diet: short-term metabolic efficacy versus longer-term limitations. Nutrients. 2021;13(4):1187. doi:10.3390/nu13041187
28. Valenzuela PL, Castillo-Garcia A, Lucia A, Naclerio F. Effects of combining a ketogenic diet with resistance training on body composition, strength, and mechanical power in trained individuals: a narrative review. Nutrients. 2021;13(9):3083. doi:10.3390/nu13093083
29. Wilson JM, Lowery RP, Roberts MD, et al. Effects of ketogenic dieting on body composition, strength, power, and hormonal profiles in resistance training men. J Strength Cond Res. 2020;34(12):3463–3474. doi:10.1519/JSC.0000000000001935
30. Antonio J, Candow DG, Forbes SC, Ormsbee MJ, Saracino PG, Roberts J. Effects of dietary protein on body composition in exercising individuals. Nutrients. 2020;12(6):1890. doi:10.3390/nu12061890
31. Tragni E, Vigna L, Ruscica M, et al. Reduction of cardio-metabolic risk and body weight through a multiphasic very-low calorie ketogenic diet program in women with overweight/obesity: a study in a real-world setting. Nutrients. 2021;13(6):1804. doi:10.3390/nu13061804
32. Dowis K, Banga S. The potential health benefits of the ketogenic diet: a narrative review. Nutrients. 2021;13(5):1654. doi:10.3390/nu13051654
33. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336–347. doi:10.1111/dom.12824
34. Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102(1):49–63. doi:10.1016/j.mcna.2017.08.006
35. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi:10.1210/jc.2014-3415
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

