Back to Journals » Clinical Epidemiology » Volume 14
A Novel Biomarker Scoring System Alone or in Combination with the GRACE Score for the Prognostic Assessment in Non-ST-Elevation Myocardial Infarction
Authors Yao Y, Shao C, Li X, Wang Z, Zuo C, Yan Y, Lv Q
Received 8 April 2022
Accepted for publication 21 July 2022
Published 2 August 2022 Volume 2022:14 Pages 911—923
DOI https://doi.org/10.2147/CLEP.S370004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lars Pedersen
Yao Yao,1,* Chunlai Shao,2,* Xiaoye Li,1 Zi Wang,1 Chengchun Zuo,1 Yan Yan,3 Qianzhou Lv1
1Department of Pharmacy, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of Cardiology, The Second Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 3Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Yan; Qianzhou Lv, Zhongshan Hospital, 180 Fenglin Road, Shanghai, 200032, People’s Republic of China, Tel +86 13916088938, Fax +86 021-64041990, Email [email protected]; [email protected]
Purpose: The Global Registry of Acute Coronary Events (GRACE) score has proven value in predicting short-term prognosis in non-ST-elevation myocardial infarction (NSTEMI), but it has only moderate discrimination for long-term outcomes. The purpose of this study is to develop and test a multi-biomarker score for better risk stratification and indication of 2-year risk in patients with NSTEMI.
Patients and Methods: A total of 6076 consecutive patients with NSTEMI (66 [59– 73] years, 73.1% males) admitted at Zhongshan Hospital, Fudan University were collected in this observational, prospective study between 2012 and 2018 with a 24-month follow-up. The primary endpoint was all-cause death and non-fatal major adverse cardiac events (MACE). A biomarker score ranged from 0 to 12 was constructed. The predictive power of the biomarker score was evaluated alone or combined with the GRACE score by C-statistic, net reclassification index (NRI) and integrated discrimination index (IDI).
Results: During a 2-year follow-up, all-cause death occurred in 159 patients (2.6%), and non-fatal MACEs were presented in 709 patients (11.7%). When added to the GRACE score, the biomarker score demonstrated better prognostic accuracy, patient reclassification and risk discrimination for both mortality and non-fatal MACEs at 2 years by improving the C-statistic from 0.714 (0.671– 0.756) and 0.623 (0.600– 0.646) to 0.851 (0.820– 0.882) and 0.721 (0.702– 0.741) with NRI > 25% (P< 0.001) and IDI > 0.30 (P< 0.001).
Conclusion: The single use of biomarker score could markedly enhance the prognostic value of concurrent risk stratification tools for 2-year mortality and non-fatal MACEs in NSTEMI. The GRACE score with incorporation of the biomarker score led to more accurate risk reclassification and warrants more consideration in further NSTEMI management.
Keywords: risk stratification, prognosis, net reclassification improvement, integrated discrimination improvement
Introduction
Acute coronary syndrome (ACS) refers to an array of diseases, including unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction. Incidence of UA and NSTEMI accounts for 70% of all patients, with over 625,000 new cases annually in the United States.1 With the advancement of percutaneous coronary revascularization and widespread use of evidence-based antiplatelet and secondary prevention medications, outcomes following myocardial infarction have improved.2 However, there remains an approximately 5% one-year residual rate of mortality after the first diagnosis of NSTEMI.3,4
Risk stratification to identify high-risk patients after discharge can optimize post-ACS therapy.5 To simplify the process of risk prediction, several post-ACS scoring systems have been derived to evaluate short-term or long-term cardiovascular risk in NSTEMI. The Thrombolysis in Myocardial Infarction (TIMI) risk score is an easy and valid tool to predict 30-day and 1-year mortality.6,7 However, the lack of weighting for the risk indicators leads to misclassification errors.8 The use of the Global Registry of Acute Coronary Events (GRACE) score in NSTEMI patients has a class IA recommendation for guiding prognosis in current guidelines.1,9,10 An obvious drawback was that the results of GRACE predicted only 6-month mortality and were published over 10 years ago, which is not necessarily representative of current clinical practice.11
While most prior risk-scoring models give a bias towards disease dimensions related to outcome in NSTEMI, biomarkers constitute an integral part in risk assessment.12 The levels of different biomarkers indicate different pathophysiological processes involving inflammatory conditions, cardiac dysfunction, coagulation function, etc. Many types of biomarkers have been investigated, and some of them have been validated in large clinical trials, which offers recommendations for a re-evaluation of the risk prediction tools with additional multimarker-integrated approach to assess the long-term prognostic utility.13,14
Therefore, we developed a multi-biomarker score with the biomarkers collected from the database of this single-center study to provide relatively easy and convenient means to predict 2-year incidence of all-cause death and non-fatal major adverse cardiac events (MACE) in NSTEMI patients. We also investigated the incremental value of this biomarker score of risk-stratification by comparing with the GRACE score.
Materials and Methods
Study Design and Population
The study was a prospective single-center observational study. A total of 6695 consecutive NSTEMI inpatients were screened, with 6076 patients included in the final analysis (Supplementary Figure 1 shows a screening flowchart). All treatment and management decisions were at the discretion of the attending physicians with a preferred practice of coronary angiography and revascularization. Eligible patients were aged ≥18 at the time of hospital presentation and had a diagnosis of NSTEMI in the presence of at least 10-minute acute chest pain at rest with one positive serum biomarker of myocardial necrosis and without new ST-segment elevation in electrocardiography. Only the first NSTEMI admission was included for patients presenting more than one ACS admission during the observation period. Exclusion criteria were incomplete records, incomplete follow-up of less than 2 years or mortality during hospital stay or within 30 days after discharge. The ethics committee of Zhongshan Hospital, Fudan University reviewed and approved the study protocol in compliance with the Helsinki Declaration. Written informed consents for collection and analysis of biological specimens were obtained from all participants on admission.
Data Collection
Baseline data were collected for each patient through electronic medical records. Demographic characteristics include age, gender and body mass index (BMI), which was calculated as weight (kg) ÷ height (m)2. We defined smokers as currently smoking or having quit for less than 6 months. Smoking status, comorbidities, family history, vital signs and Killip class were recorded at admission. According to the results of coronary angiography or coronary computed tomography angiography, number of coronary vessels with stenosis ≥50% was documented as 1-, 2- or 3-vessel disease. Cardiac function by transthoracic echocardiography was assessed before discharge. Baseline medications refer to the discharge prescriptions.
Fasting blood samples were collected on average 10 hours after admission in the early morning from an antecubital vein into citrate vacuum tubes for coagulation function and vacutainer tubes containing EDTA for determination of blood routine, hepatic function, renal function, lipid profiles and other biochemical indicators. All analyses were performed on plasma with the exception of the whole blood for blood routine and hemoglobin A1c (HbA1C) examination. The blood samples were promptly centrifuged at 4000×g for 10 minutes at 4°C. All the tests were conducted in the hospital laboratory department, which acquired ISO 15189 certification. A Sysmex XS 500i hematology analyser (Sysmex, Kobe, Japan) was used to examine blood routine. Biochemistry indices were detected by a Hitachi 7600–120 automated biochemistry analyzer (Hitachi, Tokyo, Japan). Coagulation test were performed on the STA-R Evolution Coagulation Analyzer (Stago, Asnières sur Seine, France). Cardiac troponin T (cTnT) was measured using a 5th generation Roche Elecsys highly sensitive assay (Roche Diagnostics, Mannheim, Germany) with a lower limit of 0.03 ng/mL and an upper limit of 10 ng/mL. The assay range for N-terminal pro-B-type natriuretic peptide (NT-proBNP) was 5–35000 pg/mL by using the Roche Elecsys NT-proBNP electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). Reference intervals of biomarkers are listed in Supplementary Table 1 and were determined by the hospital laboratory department and the manufacturers.
Follow-Up and Study Outcomes
All patients were followed up once a month for two years or until death after discharge through outpatient interview or telephone contact performed by a trained team. After the study period, follow-up information from 97.5% of the patients was obtained. The primary endpoint was the composite of all-cause death and non-fatal major adverse cardiovascular events (MACE) which included recurrent ACS, repeated coronary revascularization, ischemic or hemorrhagic stroke and unscheduled readmission greater than 24 hours by new/worsening heart failure or arrhythmia with corresponding symptoms and objective signs. The observation unit was recorded as person-month. The results of risk were converted into rates of events per person-year.
Calculation of the GRACE and TIMI Score
The GRACE score is derived from eight clinical variables which are age, heart rate, systolic blood pressure, serum creatinine concentration, Killip class, cardiac arrest at admission, presence of ST-segment deviation and elevated troponin (or other cardiac necrosis biomarkers). The calculation of the GRACE score was performed online (available at http://www.outcomes-umassmed.org/grace) at admission for each patient.
The TIMI score for UA/NSTEMI was based on 7 indicators ascertained upon admission ranging from 0 to 7 points: age ≥65, ≥3 risk factors for coronary artery disease, prior coronary stenosis of 50% or more, ST-segment deviation on electrocardiogram at presentation, at least 2 anginal events in prior 24 hours, use of aspirin in prior 7 days and elevated serum cardiac markers.5
Statistical Analysis
Normality was examined using Kolmogorov–Smirnov test for all data, and equality of variances was assessed by Levene’s test. Quantitative data were reported as means with standard deviations (mean ± SD) or median with interquartile range (IQR) compared by Student t-test or Mann–Whitney U-test, while qualitative data were presented as absolute numbers and percentages compared by chi-square test or Fisher’s exact probability test. For skewed distribution data, 95% confidence intervals (CIs) of median difference were calculated by the Hodges–Lehmann estimate based on the Mann–Whitney U-test.
The univariate Cox regression model was applied to explore the effects of potential biomarkers. We screened the biomarkers with Walt test P<0.05 as candidates for multivariate analyses, by which independent prognostic biomarkers were identified using a backward stepwise Cox regression method with adjustment for GRACE scores and cTnT. The selected biomarker levels were stratified by quintiles. Some biomarker levels, for example, eGFR and HDL-C, correlated inversely with risk of MACEs, and we empirically regarded the highest quintile as the reference group. Hazard ratios (HRs) for individual biomarker levels were determined using the Cox proportional hazards regression model after controlling for GRACE scores and cTnT. A biomarker score was established by converting the statistically significant HRs to the nearest integer values as follows: biomarkers with an adjusted HR of 1.2 to 1.499 were assigned a weight of 1, biomarkers with an adjusted HR of 1.5 to 2.499 were assigned a weight of 2 and biomarkers with an adjusted HR of 2.5 or higher were assigned a weight of 3. These scores were calculated as the sum to generate a final biomarker score.
Receiver-operating characteristic curves were plotted to assess the prognostic accuracy of the biomarker and GRACE score for death and non-fatal MACEs by comparing the C-index. To test the internal validity of the model, a bootstrap resampling approach with 200 replications was carried out to obtain optimism corrected estimates of the C-index. We calculated the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to assess model discrimination. The NRI is a measure of how well the model correctly assigns individuals to their corresponding groups. As no threshold was recommended, categorical NRIs were applied with risk categories in accordance with the distribution of the cumulative incidence of primary outcomes.15 NRI is sensitive to the choice of threshold, thus the continuous NRI was examined, which represents any change of risk increase or decrease in event and nonevent subsets. The IDI quantifies changes in average sensitivity and specificity between two models. Model goodness of fit was evaluated by Hosmer–Lemeshow test and was shown by calibration plots graphing the observed events against predictive outcomes.
Statistical analyses were performed using SPSS version 26.0 or R Studio version 2021.09.0 with R version 4.0.5.
Results
Study Population
A total of 6076 patients diagnosed as NSTEMI-ACS from January 2012 to December 2018 were included in the study and followed-up for a mean of 22.7 months. A total of 159 (2.6%) all-cause deaths and 709 (11.7%) non-fatal MACEs were observed during the 24-month follow-up. Baseline characteristics of patients with and without primary endpoints are shown in Table 1. Over 70% (73.1% in all cohort, 72.4% in no-event group and 76.9% in event group) of the population was male with a median age of 66 [59–73] at time of admission. Patients with primary endpoints were more likely to have comorbidities, present Killip class III or IV and have multivessel lesions. TIMI scores and GRACE scores in the event group were significantly higher compared to the no-event group (P<0.001). Differences among biomarkers were all statistically significant except detectable levels of cTnT (P<0.05).
 |
Table 1 Baseline Characteristics and Biomarkers of NSTEMI Patients |
Model Development
Table 2 summarizes the derivation of the biomarker score. As shown in the table, all predictors had a significant relationship with the primary outcome, and were subjected to multivariate stepwise backward Cox regression analysis with adjustment for GRACE score and cTnT, leading to the removal of white blood cell count (WBC), platelet count (PLT), activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), international normalized ratio (INR), D-D (D-dimer), high-sensitivity C-reactive protein (hsCRP), HbA1c, estimated glomerular filtration rate (eGFR), albumin (ALB) and total cholesterol (TC) from the final model.
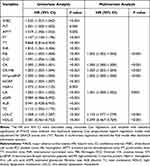 |
Table 2 Cox Proportional Hazard Regression Analyses for the Composite Point of All-Cause Death and Non-Fatal MACEs at 2 Years |
Therefore, the statistically significant biomarkers included hemoglobin (Hb), fibrinogen (FIB), creatine kinase (CK), CK-MB, NT-proBNP, uric acid (UA), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) and were quintiled respectively, and the association between biomarker levels and the composite endpoint are reported in Supplementary Figure 2. A 1.2-fold to 2.7-fold gradient of risk was observed comparing the highest to lowest concentrations across groups of FIB, CK, CK-MB, NT-proBNP, UA and LDL-C. There remained a 1.7-fold gradient risk in the lowest concentration group of HDL-C in comparison with the highest concentration. A biomarker score was constructed by assigning tiered integer values to each significant biomarker group and was calculated by summing up the values with a range from 0 to 12 for each patient, of which the indicators included FIB, CK, CK-MB, NT-proBNP, UA, LDL-C and HDL-C (Table 3).
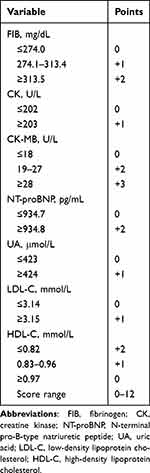 |
Table 3 Final Biomarker Risk Score |
Distributions of Biomarker Scores and GRACE Scores
Figure 1A and 1B show the distributions of biomarker scores and GRACE scores, respectively. The rate of non-fatal MACE ranged from 2.3% per month in patients with a biomarker score of 0 to 27.5% per month in patients with a biomarker score of ≥11, which was approximately 12-fold greater. The rate of death had a similar trend in elevated biomarker scores, ranging from 0.3% to 19.3% (Figure 1C). Although the rates of the same endpoints increased with higher levels of GRACE scores, a greater relative increase in risk was identified in biomarker scores (Figure 1D).
Prognostic Discrimination and Reclassification
When each single-item score in the biomarker scoring system was individually added to the GRACE score, biomarkers significantly improved the prognostic accuracy for risk of both mortality and non-fatal MACEs (Table 4). Biomarker scores alone showed greater C-statistic of 0.836 (95% CI, 0.801–0.871) for death and 0.692 (95% CI, 0.674–0.719) for non-fatal MACEs compared to GRACE scores with C-statistic of 0.714 (95% CI, 0.671–0.756) and 0.623 (95% CI, 0.600–0.646), respectively. Combining biomarker scores with GRACE scores incrementally elevated the C-statistic to 0.851 (95% CI, 0.820–0.822) for predicting mortality and 0.721 (0.702–0.741) for non-fatal MACEs.
 |
Table 4 Prognostic Discrimination |
NRI categories were based on risk of all-cause death (<2%, 2% to 8%, >8%) and non-fatal MACEs (<10%, 10% to 20%, >20%) at 2 years. The newly created biomarker score improved discrimination ability for primary endpoints in categorical NRI, continuous NRI and IDI individually or in combination with GRACE scores. Only the single-item scores of CK-MB and BNP improved all metrics of discrimination for the primary outcomes. Simultaneously categorized risk was based on the threshold mentioned above to show annual rates of primary endpoints according to GRACE scores and biomarker scores (Supplementary Figure 3). Generally, biomarker scores observed more positive reclassification of risk than negative reclassification, reflected by identifying more patients with a GRACE score ≤116 who had a higher biomarker score than patients with a GRACE score ≥140 who had a lower biomarker score. Specifically, the biomarker scoring system reclassified 208 GRACE low-risk patients to high-risk groups versus 108 moved from GRACE high-risk group to biomarker low-risk group in predicting mortality (Supplementary Figure 3A), while 236 were reclassified to higher risk versus 149 reclassified to lower risk in prediction of MACEs (Supplementary Figure 3B).
Model Validation
In addition to C-statistic, calibration plots for internal validation of biomarker scores are presented in Supplementary Figure 4; both showed good fit between the predicted and observed 2-year risk of primary outcomes. All points matched close to the 45° ideal line with a calibration bootstrap slope 1.03 (95% CI, 0.97–1.09) reflecting a small degree of overestimation of mortality (Supplementary Figure 4A). Similarly, the calibration slope for non-fatal MACEs was 1.11 (95% CI, 0.95–1.27) (Supplementary Figure 4B).
Discussion
In the present study, we developed a biomarker score that accurately predicted 2-year prognosis and showed good discrimination in risk stratification for both mortality and non-fatal MACE of NSTEMI patients both alone and in combination with the GRACE score. The score comprises seven readily obtainable indicators upon admission: FIB, CK, CK-MB, BNP, UA, LDL-C and HDL-C, which is simple to use in clinical practice. The biomarker scores stratify patients into 3 distinctive risk groups (low risk: 0–2; intermediate risk: 3–4; high risk: 5–8) and reclassified more patients from low-risk to high-risk than those inappropriately moved from high-risk to low-risk.
Management decisions in NSTEMI should be based on risk stratification to avoid a subjective judgement from physicians, for example, underestimating the risk among high-risk populations leading to inactive treatment. The GRACE score performs well in individualized patient risk assessment to guide the invasive timing in hospital and has been established as the gold standard score for ACS management.1,9,10,16 The GRACE score was first designed and validated to predict 6-month post-ACS discharge death with C-Statistic >0.75,11 and was generalized to predict mortality beyond 6 months with C-Statistic=0.81 at 2 years.17 The poor predictive performance (C-Statistic=0.62) of the GRACE score in non-fatal MACE and the moderate performance in predicting all-cause mortality (C-Statistic=0.71) in our study were comprehensible with several explanations accounting for the difference. First, we did not include the patients who died in hospital and within 30 days after discharge. Both TIMI and GRACE risk scores have superior prediction for in-hospital and 30-day post-discharge mortality, while the focus of the study is prognosis assessment and therapeutic decision-making for a longer term.1,6,9,10 Second, our study population included only NSTEMI eastern Chinese Han patients. This may indicate that the biomarker score derived from this study is more specific to NSTEMI than the current risk prediction tools for all types of ACS. Third, the proportion of Killip III/IV patients was relatively lower in our database. Additionally, the guideline-directed medical treatment has improved prognosis significantly and reduced the mortality rates.18,19 Non-fatal MACE is also an important outcome for risk stratification since the second hit for the heart remains a leading cause for death.20 A single biomarker may not be enough to determine prognosis, so we screened a series of potential biomarkers. The integrated biomarkers shared links which simultaneously evaluated the physiological and pathological processes of NSTEMI.21
We used contemporary statistical methods, IDI and NRI, to examine the improvement of prognostic assessment and reclassification in the study. We found that each biomarker in the newly established score has added value for determination of prognosis.
Acute exacerbation of ACS involves severe inflammatory response.22 FIB is a liver protein synthesized increasingly in response to circulating proinflammatory cytokines.23 FIB plays a critical role in determination of plasma viscosity, red cell aggregation as well as platelet activation, and it is recognized as a major factor in the initiation and progression of atherogenesis.24,25 There is evidence that plasma FIB levels can be used as an independent predictor of MACEs, and its combination with ALB, D-D and other hematological indicators also suggests a strong predictive value.26–29 It is commonly assumed that CK-MB remains a reliable marker of cardiomyocyte damage and has been shown to predict elevated MACEs in NSTEMI patients after discharge.30–32 As one of the three CK isoenzymes, CK-MB is detectable in plasma 4–8 hours following onset of myocardial infarction and peaks at 18–24 hours.33 Elevated CK-MB levels are related to a worse prognosis in our study, and a meta-analysis also indicates that a greater increase (>5× the upper limit of normal) in CK-MB causes significant improvements in mortality, which may be explained by the myocyte apoptosis affecting contractile function.34 CK catalyzes the reversible conversion of creatine and ATP into phosphocreatine and ADP.35 The diagnostic and prognostic role of CK in NSTEMI has been controversial. Some studies have suggested the elimination of CK assays to contain costs.36,37 However, our results show that CK has a positive prognosis value after adjusting for a wide range of biomarkers. Several studies have demonstrated similar results and emphasized the role of both CK and CK-MB reflecting the severity of myocardial injury.38,39 NT-proBNP is a highly sensitive marker of left ventricular dysfunction and fluid retention especially in patients with heart failure. The level of NT-proBNP relating to MACEs in NSTEMI may indicate the presence of comorbidities and has been a strong marker of long-term outcomes in an increasing number of studies.40 UA is the terminal oxidation product of purine catabolism.41 In addition to acting as a surrogate marker of dehydration in serum hyperosmolarity, it is also known as an oxidative stress marker which is presented in linear correlation to the high levels of oxidation LDL (ox-LDL) and CRP.42–44 Real-world studies have validated the relationship of high levels of UA and MACEs in patients with ACS and suggested UA as a new prognostic marker.45–47 Studies have long shown that lipids play an essential role in the development of coronary atherosclerosis beyond doubt.48 High levels of LDL have adverse effects on coronary vessels, while HDL presents a cardioprotective effect.49,50 Reduction of LDL and elevation of HDL can improve outcomes and prevent relapse of MACEs.51 Many other biomarkers suggesting peripheral dyslipidemia, such as lipoprotein(a), small dense LDL and ox-LDL have been measured and shown predictive effects.52,53
In our cohort, no significant difference was observed in the baseline medications of renin-angiotensin antagonists and statins between no-event group and event group. This might be due to the relatively low-risk patients in our study and the attending physicians prescribing the medications rigorously following the guidelines. Besides, it is probably related with patient compliance, which was not taken into consideration. We are cautious not to overstate this finding, because the reasons remain to be determined.1,10
Some limitations in this study warrant acknowledgement. First, the non-interventional nature of this study may cause incomplete data collection and the loss of sample size. Second, this is a single-center study mainly based in Shanghai, a regional area in China. The quantitative findings in our population might not be generalizable to the entire NSTEMI population. Besides, the biomarker score we created in this study has not been externally validated, which requires further studies across different regions during different time periods. Apart from the enhancement in the convenience of clinical application, the quintiled biomarkers and GRACE scores used in the study may lose information compared to the continuous form of parameters. Finally, there may be incomplete confounders, such as mean platelet volume, a thrombogenic index for immature platelets, which is related to the prognosis.54 Further consideration should be given to novel biomarker testing to address the incremental prognostic benefit from that of current biomarker scores.
Conclusion
In conclusion, this easy-to-use biomarker scoring system with the incorporation of GRACE scores can quickly identify the high-risk patients. Health care workers may refer to the biomarker score during medical decision-making and initiate more intensive follow-up accordingly.
Acknowledgments
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi:10.1016/j.jacc.2014.09.017
2. Timmis A, Townsend N, Gale CP, et al. European society of cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12–85. doi:10.1093/eurheartj/ehz859
3. Lenzen MJ, Boersma E, Bertrand ME, et al. Management and outcome of patients with established coronary artery disease: the Euro heart survey on coronary revascularization. Eur Heart J. 2005;26(12):1169–1179. doi:10.1093/eurheartj/ehi238
4. Bouisset F, Ruidavets JB, Dallongeville J, et al. Comparison of short- and long-term prognosis between ST-Elevation and Non-ST-elevation myocardial infarction. J Clin Med. 2021;10:2. doi:10.3390/jcm10020180
5. Eagle KA, Ginsburg GS, Musunuru K, et al. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation. 2010;121(12):1447–1454. doi:10.1161/CIRCULATIONAHA.109.904029
6. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835–842. doi:10.1001/jama.284.7.835
7. Morrow DA, Antman EM, Giugliano RP, et al. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: an InTIME II substudy. Lancet. 2001;358(9293):1571–1575. doi:10.1016/S0140-6736(01)06649-1
8. Bawamia B, Mehran R, Qiu W, Kunadian V. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J. 2013;165(4):441–450. doi:10.1016/j.ahj.2012.12.020
9. Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333(7578):1091. doi:10.1136/bmj.38985.646481.55
10. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
11. Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–2733. doi:10.1001/jama.291.22.2727
12. Scirica BM. Acute coronary syndrome: emerging tools for diagnosis and risk assessment. J Am Coll Cardiol. 2010;55(14):1403–1415. doi:10.1016/j.jacc.2009.09.071
13. Widera C, Pencina MJ, Meisner A, et al. Adjustment of the GRACE score by growth differentiation factor 15 enables a more accurate appreciation of risk in non-ST-elevation acute coronary syndrome. Eur Heart J. 2012;33(9):1095–1104. doi:10.1093/eurheartj/ehr444
14. Lindholm D, James SK, Gabrysch K, et al. Association of multiple biomarkers with risk of all-cause and cause-specific mortality after acute coronary syndromes: a secondary analysis of the PLATO biomarker study. JAMA Cardiol. 2018;3(12):1160–1166. doi:10.1001/jamacardio.2018.3811
15. Pencina MJ, D’Agostino RB
16. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353. doi:10.1001/archinte.163.19.2345
17. Tang EW, Wong CK, Herbison P. Global registry of acute coronary events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153(1):29–35. doi:10.1016/j.ahj.2006.10.004
18. Goldberg RJ, Spencer FA, Yarzebski J, et al. A 25-year perspective into the changing landscape of patients hospitalized with acute myocardial infarction (the worcester heart attack study). Am J Cardiol. 2004;94(11):1373–1378. doi:10.1016/j.amjcard.2004.07.142
19. Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297(17):1892–1900. doi:10.1001/jama.297.17.1892
20. Kühl JT, Møller JE, Kristensen TS, Kelbæk H, Kofoed KF. Left atrial function and mortality in patients with NSTEMI an MDCT study. JACC Cardiovasc Imaging. 2011;4(10):1080–1087. doi:10.1016/j.jcmg.2011.08.008
21. Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105(15):1760–1763. doi:10.1161/01.CIR.0000015464.18023.0A
22. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136(12):1155–1166. doi:10.1161/CIRCULATIONAHA.117.029870
23. de Moerloose P, Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35(4):356–366. doi:10.1055/s-0029-1225758
24. Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29(5):435–450.
25. Tousoulis D, Papageorgiou N, Androulakis E, Briasoulis A, Antoniades C, Stefanadis C. Fibrinogen and cardiovascular disease: genetics and biomarkers. Blood Rev. 2011;25(6):239–245. doi:10.1016/j.blre.2011.05.001
26. Shi Y, Wu Y, Bian C, Zhang W, Yang J, Xu G. Predictive value of plasma fibrinogen levels in patients admitted for acute coronary syndrome. Tex Heart Inst J. 2010;37(2):178–183.
27. Karahan O, Acet H, Ertaş F, et al. The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am J Emerg Med. 2016;34(6):1037–1042. doi:10.1016/j.ajem.2016.03.003
28. He D, Jiao Y, Yu T, et al. Prognostic value of fibrinogen-to-albumin ratio in predicting 1-year clinical progression in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention. Exp Ther Med. 2019;18(4):2972–2978. doi:10.3892/etm.2019.7890
29. Zhang L, Xu C, Liu J, et al. Baseline plasma fibrinogen is associated with haemoglobin A1c and 2-year major adverse cardiovascular events following percutaneous coronary intervention in patients with acute coronary syndrome: a single-centre, prospective cohort study. Cardiovasc Diabetol. 2019;18(1):52. doi:10.1186/s12933-019-0858-5
30. Galla JM, Mahaffey KW, Sapp SK, et al. Elevated creatine kinase-MB with normal creatine kinase predicts worse outcomes in patients with acute coronary syndromes: results from 4 large clinical trials. Am Heart J. 2006;151(1):16–24. doi:10.1016/j.ahj.2005.01.045
31. Jernberg T, Lindahl B, James S, Ronquist G, Wallentin L. Comparison between strategies using creatine kinase-MB(mass), myoglobin, and troponin T in the early detection or exclusion of acute myocardial infarction in patients with chest pain and a nondiagnostic electrocardiogram. Am J Cardiol. 2000;86(12):1367–1371, a1365. doi:10.1016/S0002-9149(00)01245-5
32. Chin CT, Wang TY, Li S, et al. Comparison of the prognostic value of peak creatine kinase-MB and troponin levels among patients with acute myocardial infarction: a report from the acute coronary treatment and intervention outcomes network registry-get with the guidelines. Clin Cardiol. 2012;35(7):424–429. doi:10.1002/clc.21980
33. Hedges JR. The role of CK-MB in chest pain decision-making. J Accid Emerg Med. 1995;12(2):101–106. doi:10.1136/emj.12.2.101
34. Bostan MM, Stătescu C, Anghel L, Șerban IL, Cojocaru E, Sascău R. Post-myocardial infarction ventricular remodeling biomarkers-the key link between pathophysiology and clinic. Biomolecules. 2020;10:11. doi:10.3390/biom10111587
35. Sumien N, Shetty RA, Gonzales EB. Creatine, creatine kinase, and aging. Subcell Biochem. 2018;90:145–168.
36. Le RD, Kosowsky JM, Landman AB, Bixho I, Melanson SE, Tanasijevic MJ. Clinical and financial impact of removing creatine kinase-MB from the routine testing menu in the emergency setting. Am J Emerg Med. 2015;33(1):72–75. doi:10.1016/j.ajem.2014.10.017
37. Wiens EJ, Arbour J, Thompson K, Seifer CM. Routine creatine kinase testing does not provide clinical utility in the emergency department for diagnosis of acute coronary syndromes. BMC Emerg Med. 2019;19(1):37. doi:10.1186/s12873-019-0251-4
38. Halkin A, Stone GW, Grines CL, et al. Prognostic implications of creatine kinase elevation after primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2006;47(5):951–961. doi:10.1016/j.jacc.2005.12.003
39. Nienhuis MB, Ottervanger JP, de Boer M-J, et al. Prognostic importance of creatine kinase and creatine kinase–MB after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am Heart J. 2008;155(4):673–679. doi:10.1016/j.ahj.2007.11.004
40. Jernberg T, James S, Lindahl B, Stridsberg M, Venge P, Wallentin L. NT-ProBNP in non–ST-elevation acute coronary syndrome. J Card Fail. 2005;11(5):S54–S58. doi:10.1016/j.cardfail.2005.04.010
41. Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–163. doi:10.1016/j.cca.2018.05.046
42. Hahn K, Kanbay M, Lanaspa MA, Johnson RJ, Ejaz AA. Serum uric acid and acute kidney injury: a mini review. J Adv Res. 2017;8(5):529–536. doi:10.1016/j.jare.2016.09.006
43. Magnoni M, Berteotti M, Ceriotti F, et al. Serum uric acid on admission predicts in-hospital mortality in patients with acute coronary syndrome. Int J Cardiol. 2017;240:25–29. doi:10.1016/j.ijcard.2017.04.027
44. Cicero AF, Rosticci M, Cagnati M, et al. Serum uric acid and markers of low-density lipoprotein oxidation in nonsmoking healthy subjects: data from the brisighella heart study. Pol Arch Med Wewn. 2014;124(12):661–668. doi:10.20452/pamw.2548
45. Trkulja V, Car S. On-admission serum uric acid predicts outcomes after acute myocardial infarction: systematic review and meta-analysis of prognostic studies. Croat Med J. 2012;53(2):162–172. doi:10.3325/cmj.2012.53.162
46. Yan L, Liu Z, Zhang C. Uric acid as a predictor of in-hospital mortality in acute myocardial infarction: a meta-analysis. Cell Biochem Biophys. 2014;70(3):1597–1601. doi:10.1007/s12013-014-0101-7
47. Li L, Zhao M, Wang C, et al. Early onset of hyperuricemia is associated with increased cardiovascular disease and mortality risk. Clin Res Cardiol. 2021;110(7):1096–1105. doi:10.1007/s00392-021-01849-4
48. Claessen BE, Guedeney P, Gibson CM, et al. Lipid management in patients presenting with acute coronary syndromes: a review. J Am Heart Assoc. 2020;9(24):e018897. doi:10.1161/JAHA.120.018897
49. Michos ED, McEvoy JW, Blumenthal RS. Lipid management for the prevention of atherosclerotic cardiovascular disease. N Engl J Med. 2019;381(16):1557–1567. doi:10.1056/NEJMra1806939
50. Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. 2017;16(1):s27–s42. doi:10.5604/01.3001.0010.5495
51. Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges. Circ Res. 2016;118(4):531–534. doi:10.1161/CIRCRESAHA.116.308334
52. O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483–1492. doi:10.1161/CIRCULATIONAHA.118.037184
53. Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52(2):70–85. doi:10.3109/10408363.2014.992063
54. Faber J, Hvas AM, Kristensen SD, et al. Immature platelets and risk of cardiovascular events among patients with ischemic heart disease: a systematic review. Thromb Haemost. 2021;121(5):659–675. doi:10.1055/s-0040-1721386
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

