Back to Journals » Clinical Ophthalmology » Volume 10
A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease
Authors Friedman N, Butron Valdez K, Robledo Silva N, Loudin J, Baba S, Chayet A
Received 2 December 2015
Accepted for publication 12 February 2016
Published 4 May 2016 Volume 2016:10 Pages 795—804
DOI https://doi.org/10.2147/OPTH.S101716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Arturo Chayet
Views: 22342
Neil J Friedman,1 Karla Butron,2 Nora Robledo,2 James Loudin,3 Stephanie N Baba,3 Arturo Chayet2
1Department of Ophthalmology, Stanford University, Mid-Peninsula Ophthalmology Medical Group, Palo Alto, CA, USA; 2Codet Vision Institute, Tijuana, Mexico; 3Oculeve, Inc., South San Francisco, CA, USA
Background: Dry eye disease (DED), a chronic disorder affecting the tear film and lacrimal functional unit, is a widely prevalent condition associated with significant burden and unmet treatment needs. Since specific neural circuits play an important role in maintaining ocular surface health, microelectrical stimulation of these pathways could present a promising new approach to treating DED. This study evaluated the efficacy and safety of nasal electrical stimulation in patients with DED.
Methods: This prospective, open-label, single-arm, nonrandomized pilot study included 40 patients with mild to severe DED. After undergoing two screening visits, enrolled subjects were provided with a nasal stimulation device and instructed to use it at home four times daily (or more often as needed). Follow-up assessments were conducted up to day 180. The primary efficacy endpoint was the difference between unstimulated and stimulated tear production quantified by Schirmer scores. Additional efficacy endpoints included change from baseline in corneal and conjunctival staining, symptoms evaluated on a Visual Analog Scale, and Ocular Surface Disease Index scores. Safety parameters included adverse event (AE) rates, visual acuity, intraocular pressure, slit-lamp biomicroscopy, indirect ophthalmoscopy, and endoscopic nasal examinations.
Results: Mean stimulated Schirmer scores were significantly higher than the unstimulated scores at all visits, and corneal and conjunctival staining and symptom scores from baseline to day 180 were significantly reduced. No serious device-related AEs and nine nonserious AEs (three device-related) were reported. Intraocular pressure remained stable and most subjects showed little or no change in visual acuity at days 30 and 180. No significant findings from other clinical examinations were noted.
Conclusion: Neurostimulation of the nasolacrimal pathway is a safe and effective means of increasing tear production and reducing symptoms of dry eye in patients with DED.
Keywords: keratoconjunctivitis sicca, neuromodulation, neurostimulation, nasolacrimal reflex, tear production, ocular staining
Introduction
Chronic dry eye disease (DED) is a multifactorial disorder of the tears and ocular surface, which results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface as the disease progresses.1 The condition is accompanied by increased osmolarity of the tear film and ocular surface inflammation.1 DED is a leading cause of eye discomfort and morbidity globally. Approximately 25 million Americans suffer from DED, and symptoms of the disease are among the leading causes for patient visits to ophthalmologists and optometrists in the US, accounting for ~25% of all patients who seek eye care.2–5 It is estimated that ~5 million patients aged 50 years and older in the US have moderate to severe DED, and millions more experience less severe episodic symptoms of dry eye.3,6,7 The severity and prevalence of DED in the general population increases with age and is particularly common in postmenopausal women and in individuals aged 65 years and older. As a consequence, the prevalence of the disease and its associated burden are expected to increase with continued growth of aging populations.5,7 DED is associated with significant impact on visual function, including reading and driving,8 as well as daily activities, social and physical functioning, workplace productivity, and quality of life.9,10 Additionally, the disease poses a significant economic burden on both patients and health care delivery organizations as a result of the direct and indirect costs related to increased health care utilization, missed school and work, and leisure and quality-of-life issues.5,11,12
DED is associated with a dysfunction of various components of the lacrimal functional unit (LFU), an integrated system comprising the ocular surface (tear film, corneal and conjunctival epithelia with mucin-producing goblet cells), meibomian glands, lacrimal glands, and the various neural pathways that connect them.1,3,13 The LFU controls the response to internal and external stimuli and acts to preserve the integrity and function of the ocular surface.3,13 The pathogenesis of DED represents a complex and multifactorial series of events that interact to promote a vicious self-sustaining cycle of inflammation, goblet cell dysfunction/mucin loss, epithelial damage, tear film instability/evaporation, and tear hyperosmolarity.1 Neural pathways play an important role in maintaining the integrity of the cornea and tear film, and optimal functionality of these circuits is essential for the maintenance of a healthy ocular surface.3,7 The neural pathways regulating the LFU include afferent sensory nerves in the cornea and conjunctiva which, when activated, result in the stimulation of efferent parasympathetic and sympathetic nerves innervating the lacrimal gland. These efferent fibers synapse directly with the lacrimal gland acinar cells and trigger the release of water, electrolytes, and proteins from the lacrimal gland onto the ocular surface.14–16 The ocular reflex pathways that drive normal tear function become disrupted in DED, in part due to damage or loss of sensory neurons in the ocular surface, which leads to further reduction in tear production, additional nerve cell loss, and exacerbation of the disease cycle.7,17,18 A second reflex pathway, the nasolacrimal reflex (NLR), is also an important contributor to normal tear physiology and is triggered by activation of trigeminal afferent nerve fibers in the nasal cavity. This reflex has been shown in a randomized and controlled clinical setting to account for 35% of basal tear secretion, which is believed to be the result of activation of the pathway by nasal breathing.19 Activation of the NLR can be initiated by stimulating the anterior ethmoidal nerve, a sensory subunit of the ophthalmic branch of the trigeminal nerve, leading to an increase in activity in the superior salivatory nucleus region of the brain, which is responsible for control of natural lacrimation. Disruption of this pathway has also been shown to be a contributing factor in DED.1,14,15,18
DED has become an increasingly pressing concern in clinical practice,20 and the vast majority of eye care practitioners are not fully satisfied with existing treatment options for the disease.21 Various guidelines for the treatment of DED have been proposed, with specific recommendations varying depending on the staging/severity of the disease.1,2,12 There is currently no cure or single universally effective treatment for DED, and the most widely used forms of therapy tend to be palliative in nature, intended to supplement the patients’ natural tears or to improve the residence time of the limited volume of tears present. Among the more commonly used palliative options for DED, tear replacement with over-the-counter artificial tear substitutes and lubricants has limited efficacy.22,23 Similarly, punctal plugs have significant drawbacks such as the risk of complications associated with the implantation procedure, and poor retention/migration, which can lead to lacrimal obstruction and other complications including biofilm formation and infection.24–27 A significant unmet medical need remains with respect to the treatment of DED, and one of the principal shortcomings of currently available treatments for DED is their inability to directly stimulate the glands and cells to increase tear production.18
Neurostimulation, a well-established therapeutic strategy for activating peripheral nerve pathways directly to correct organ dysfunction and manage disease symptoms,28–33 represents a novel and potentially promising option for DED.18 Neurostimulation devices have been proven safe and effective for a variety of medical applications approved by the US Food and Drug Administration (FDA) and have been employed to treat a diverse array of conditions, including movement disorders, psychiatric disorders, incontinence, sensory disabilities, epilepsy, chronic headache and pain syndromes, stroke, spinal cord and traumatic brain injury, rheumatoid arthritis, and obesity.28,34 The feasibility of utilizing neurostimulation for DED is predicated on the notion that the body’s natural tear system can be upregulated by the activation of the NLR, a well-established neural pathway by which nasal stimuli promote both resting basal and bolus secretion of one or more layers of the tear film.19 The reflex plays a functional role in responding to and expelling foreign bodies or irritants from the nose by secreting tears into the nasal cavity via the nasolacrimal duct, and it serves as one of the body’s primary compensatory mechanisms for combating ocular surface dryness.
The application of electrical stimulation to sensory neurons of the nasal cavities to increase natural tear production may offer benefits for DED patients. The intranasal stimulation device under investigation in this report was designed to activate the body’s natural tear production mechanisms by delivering gentle electrical currents to sensory neurons in the nasal cavities, thereby triggering tear production. The objective of the study was to assess the ability of intranasal electrical stimulation to increase tear production and improve the signs and symptoms of DED.
Methods
Study design and participants
This study was a prospective, open-label, single-arm, nonrandomized trial conducted at Codet Vision Institute (Tijuana, BC, Mexico) between May 30, 2013 and August 20, 2014. The study population consisted of subjects with mild-to-severe DED diagnosed according to the criteria of the 2007 Report of the International Dry Eye Workshop (DEWS).1 The target for enrollment was 40 patients. Participants underwent two initial screening examinations to determine the eligibility for enrollment according to the following key inclusion criteria: male or female 18 years of age or older; Schirmer test (with topical anesthesia) value of no more than 15 mm/5 minutes at baseline (unstimulated) and retest value (during nasal stimulation with cotton swab) of at least two times or 10 mm/5 minutes higher than the unstimulated values; baseline Ocular Surface Disease Index (OSDI) score of 13 or more; normal lid anatomy, blinking function, and closure; and corrected visual acuity (VA) of 20/200 (Snellen equivalent) or better in each eye at both screening visits. The presence of ocular surface staining was not required for inclusion in the study.
Subjects were excluded if they met any of the following criteria: pregnancy or planned pregnancy/nursing at study entry; history/presence of systemic disease, including systemic allergy, acute or chronic rhinitis or sinusitis requiring treatment, myocardial infarction, unstable disease such as diabetes, hypertension, thyroid malfunction, uncontrolled autoimmune or immunocompromising disease; participation in any clinical trial with a new active substance or a new device during the past 3 months; nonstable diseases within 1 month prior to the initial screening visit; ocular disorder/infection; nasal infection or inflammation not associated with DED within 3 months prior to the initial screening visit; severe blepharitis or anterior basement membrane dystrophy of the cornea; hypersensitivity to test device materials or procedural medications; hemophilia or other disorder of coagulation; thrombocytopenia; chronic/recurrent epistaxis; chronic seasonal allergies; change within 30 days prior to first screening visit in use of topical ophthalmic or systemic medications that could affect DED; contact lens wear within 7 days prior to the first screening visit or during the duration of the study; or punctal plug use.
Subjects who, within 30 days prior to the first screening, were using ophthalmic or systemic medications that could affect DED (eg, topical cyclosporine, antihistamines, tricyclic antidepressants, anxiolytics, antimuscarinics, beta-blocking agents, phenothiazines, and steroids) were permitted to continue their use; however, changes in the use of those medications or the addition of new medications during the study were prohibited. No other experimental drug or device within 90 days prior to the first screening visit or during the course of the study was allowed. After the first screening visit, subjects using any type of ophthalmic lubricant drops were asked to discontinue their use and were provided with over-the-counter non-preserved artificial tears in unit dose vials to be administered if their DED symptoms became intolerable.
The study was conducted in accordance with Good Clinical Practice (ISO-14155) guidelines, the Declaration of Helsinki, and relevant local regulations. Institutional review board approval was obtained (Comisión de Investigación y Ética, Zona Rio, Tijuana, B.C., Mexico), and all subjects provided written informed consent. This study was registered under the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) on September 25, 2013 (ID# ACTRN12613001075774).
Test device and follow-up schedule
The test device (a prototype of the Oculeve intranasal neurostimulation device) consisted of a handheld battery-powered current-controlled neurostimulation source connected to intranasal leads. The device contacts were inserted into the nasal passage by the patient so as to make contact with the anterior nasal mucosa. The level of stimulation could be selected by the patient and at each level, the strength remained constant. Subjects were instructed on the use of the device, including insertion of the leads of the stimulator into both nostrils and application of stimulation until they felt a tickling sensation or the urge to sneeze. Subjects were then provided with a device to take home and were instructed to use it four times daily (or more often as needed).
Follow-up evaluations were scheduled for visits at days 7, 14, 30, 60, 90, and 180. Unstimulated tear production and other objective measures of DED were measured in the clinic prior to nasal stimulation at each follow-up visit. Subsequently to this, tear production during stimulation was measured at each visit.
Outcome measures
Efficacy
At each follow-up visit, tear production was assessed based on Schirmer test score results using Color Bar™ sterile test strips (Eagle Vision, Inc., Memphis, TN, USA). The test was performed under topical anesthesia with 0.5% proparacaine hydrochloride drops (provided by the participating centers), first without stimulation and then again with stimulation for the duration of the Schirmer test (5 minutes, or less if the test strip became fully wet before 5 minutes had elapsed). The difference between unstimulated and stimulated tear production served as the study’s primary efficacy endpoint. At the initial screening visit, Schirmer testing was performed before and after intranasal stimulation of the NLR with a cotton swab.
Additional efficacy endpoints included ocular surface staining, tear film break-up time (TBUT), and subject-assessments of DED symptoms and severity. Staining of the conjunctiva (nasal and temporal regions) and cornea (superior, nasal, inferior, and temporal and central regions) was assessed by lissamine green (Lissamine Green Ophthalmic Strips; Hub Pharmaceuticals LLC, Rancho Cucamonga, CA, USA) and fluorescein staining (Fluorescein GloStrips™ 1.0 mg; Amcon Laboratories, Inc., St Louis, MO, USA), respectively. For both tests, the stain strips were applied to the eye and the degree of staining was graded 1 minute later on a pictorial scale (modified Oxford Scale) with scores ranging from 0 to 5. TBUT was measured prior to stimulation, using slit-lamp biomicroscopy (10× magnification) with fluorescein and expressed as the time (in seconds) between the last complete blink and the first appearance of a growing micelle. Patients used the eight-item Dry Eye Symptom Visual Analog Scale (VAS) to rate symptoms due to ocular dryness, where 0% = no discomfort and 100% = maximal discomfort. The eight-symptom categories of the VAS were: severity of symptoms, dryness, sticky feeling, burning/stinging, foreign body sensation, blurred vision, photophobia, and pain. Patients assessed DED severity by completing the OSDI questionnaire, consisting of 12 questions rated on a 5-point scale (0= none of the time and 5= all of the time), which were compiled to generate an overall score on a scale from 0 to 100, with higher scores representing greater disability. To assess DED severity, three corneal specialists were provided with the patients’ screening results and they independently ranked each patient from I to IV using the DEWS criteria1 as a guide. The rankings for individual patients’ were averaged and rounded to obtain a composite score for each patient. Additional efficacy summaries were performed to determine the effect of stimulation on Schirmer scores in patients stratified by severity level (ie, DEWS levels I–II vs levels III–IV). Patients used diaries to record data on daily usage patterns for the stimulation device and to track responses such as ocular comfort. Patient satisfaction with the procedure and outcomes was assessed using a six-question survey, which was completed at the conclusion of the study.
Safety
The main safety outcomes of interest were the incidence of device-related adverse events (AEs) and complications. Safety assessments included corrected distance VA (CDVA); intraocular pressure (IOP) measured by applanation or noncontact tonometry; slit-lamp biomicroscopic examination of the ocular surface, anterior segment, and eyelids; indirect ophthalmoscopy with dilation for visual examination of the fundus/posterior segment of the eye; and nasal endoscopic examination. Slit-lamp assessments of conjunctival edema, tear film debris, and the eyelids (edema and erythema) were scored on a 5-point scale, ranging from none to very severe. Eyelashes were scored as either normal or abnormal. For all other safety measures (eg, slit-lamp assessment of conjunctival erythema, meibomian glands, anterior chamber, and lens), intensity/severity was graded on a 4-point scale (ie, mild, moderate, marked, or severe).
Statistical methods
Demographic, baseline, effectiveness, and safety data are represented using descriptive statistics. Data for continuous variables are represented as sample size and mean ± standard error of the mean and, where appropriate, median, minimum, and maximum. Comparisons between baseline and follow-up values were analyzed by paired t-test or Wilcoxon signed-rank test using a two-tailed distribution model with P-values <0.05 considered statistically significant. Data were analyzed for the intent-to-treat population, and missing values were not imputed.
Results
Subject disposition and demographics
Of the 50 patients screened, 41 met the eligibility criteria, one of whom failed the endoscopy screening due to severe septal deviation and never received a device application. Of the 40 enrolled subjects participating in the study, 34 completed the 180-day study period, while three subjects were lost to follow-up, and three subjects withdrew.
The baseline demographic characteristics of the study population are presented in Table 1. The mean age of the study population was 54.6±1.9 years (range, 28.0–75.0). The subjects were predominantly female (85.4%) and of Hispanic or Latino ethnicity (97.6%). A total of four patients (10%) were concurrently taking a topical anti-inflammatory agent (ie, cyclosporine 0.05%) during the study.
  | Table 1 Patient demographic characteristics |
Efficacy analysis
Primary outcome: Schirmer score
Acute tear production was measured using the Schirmer test, and the stimulated value minus the unstimulated (preadministration) value at each visit served as the study’s primary endpoint (Figure 1). The mean stimulated Schirmer score at each visit was higher than the mean unstimulated score taken at the same visit. On study day 0, the unstimulated and stimulated Schirmer scores, respectively, were 9.4±1.3 and 22.4±1.9 (a 138.3% increase) in the right eye and 10.5±1.1 and 22.5±1.8 (114.3% increase) in the left eye. After an initial period of ~2 weeks, the average increase in the stimulated Schirmer score stabilized; at day 180, the respective values were 12.7±1.7 and 19.9±2.0 (56.7% increase) in the right eye and 13.2±1.6 and 20.5±2.0 (55.3% increase) in the left eye. The difference between the stimulated and unstimulated Schirmer scores was statistically significant (P<0.001) for both eyes at all follow-up visits. Additionally, the similarity in stimulated Schirmer scores at day 0 and day 180 indicated that the enhancement of tear production persisted with chronic stimulation.
Corneal staining, conjunctival staining, and TBUT
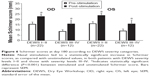
The mean changes from baseline in corneal and conjunctival staining scores by eye and visit are presented in Figures 2 and 3, respectively. Ocular surface staining scores were numerically lower at follow-up visits compared to baseline. The reduction was statistically significant at some but not all time points. However, at all study visits after 1 week, a decrease was observed in corneal and conjunctival staining scores, further demonstrating a sustained effect with chronic stimulation. At day 180, the mean change from baseline in the conjunctival staining score in the right eye was −1.8±0.5 (P=0.002) and the mean change from baseline in the corneal staining score was −1.0±0.6 (P=0.118). Values were similar in the left eye (Table 2). Ocular surface staining was not an inclusion criterion for the study, which would likely have dampened the effect of nasal stimulation on the mean change in staining for the study population as a whole. Among the subgroup of patients with baseline total corneal staining scores ≥2, the mean reduction in staining at day 180 was −2.4±1.0 for the right eye (n=18) and −2.4±1.3 for the left eye (n=19).
No statistically significant changes from baseline were observed in TBUT values with chronic nasal electrical stimulation, although TBUT was not measured in the peri-stimulation time period. Mean follow-up TBUT values in the right and left eye, respectively, were 2.8±0.3 seconds and 2.9±0.3 seconds at day 0, and 2.8±0.3 seconds and 2.7±0.3 seconds at day 180.
DED severity and symptoms
At each follow-up study visit (days 7, 14, 30, 60, 90, and 180), the mean changes from baseline in the eight VAS categories (Figure 4) were statistically significant (P<0.001). At day 180, the mean changes from baseline were as follows: severity of dry eye symptons, −38.3±3.5; eye dryness, −39.2±3.8; sticky feeling, −32.8±4.5; burning/stinging, −37.5±4.6; foreign body sensation, −37.7±4.6; blurred vision, −29.0±5.0; photophobia, −37.0±5.4; pain, −29.6±5.9.
DED severity was evaluated based on the OSDI score on a scale of 0–100, with higher scores representing greater disability. The mean OSDI score at day 0 was 69.3±2.6 and decreased to 32.6±2.8 at day 180, representing a 53.0% decline. The mean changes from baseline in OSDI scores (Figure 5) were statistically significant at each of the follow-up visits (P<0.001).
The average duration of symptom relief was 3.0±0.04 hours, which was captured in a total of 2,183 diary-recorded application days.
Efficacy by DEWS classification severity
Mean stimulated and unstimulated Schirmer scores, at day 180 stratified according to disease severity are presented in Figure 6. Data for patients with DEWS severity levels I and II and those with severity levels III and IV were pooled into two groups to generate a sufficient sample size. The results indicate that nasal stimulation led to an increase in Schirmer scores, as compared with unstimulated values, which was statistically significant both in patients with DEWS severity levels I–II (n=22) and in those with severity levels III–IV (n=12). The increases in the right and left eye ranged from 7.2 mm to 7.8 mm (47.7%–50.0%) in patients with severity levels I–II and from 6.5 mm to 7.0 mm (73.9% – 84.3%) in those with severity levels III–IV.
According to responses on the six-item survey completed at the conclusion of the study, patients were satisfied overall with the device, procedure, and outcomes (Table 3). Of the 34 respondents, 84% were moderately or highly satisfied overall, 72% indicated that they would use the device again, and 83% said they would recommend the device to others with DED.
  | Table 3 Patient survey results |
Safety analysis
According to subject-reported data from device administration diaries, the subject population reported using the device for a total of 4,575 days of stimulation during the 180 days of the study. Data for the number of stimulations per day and duration per administration were available for 3,584 of those days; the values were 3.9 per day and 4.3 minutes, respectively. As reported by patients, the typical time between consecutive applications during the day was 6 hours. The cumulative number of individual administrations in the study was 17,863.
A total of nine nonserious AEs were reported, six of which were unrelated to use of the investigational device. The three nonserious device-related AEs consisted of one migraine headache, which occurred in a subject with a 10-year history of migraine headaches and resolved in 1 day with medical intervention, one case of mild nasal discomfort following the nasal endoscopy examination, which lasted for 1 day and resolved without medical intervention (this AE was related to a study diagnostic procedure, not the investigational device), and one episode of mild nasal discomfort, which lasted for 2 days and resolved without medical intervention. The six events reported as unrelated to use of the device included one migraine headache in a subject with a prior history of migraine headaches, two tension headaches attributed to stress by the subjects, nasal discomfort attributed by the otolaryngologist to allergic rhinitis, swelling on the external skin near the bridge of the nose, and a benign lesion in the nasopharynx.
IOP, which was measured at screening, day 0, day 30, and day 180, was found to remain stable over the course of the study and was relatively unchanged from baseline. The mean IOP readings in the right and left eye, respectively, were as follows: screening visit (day -7), 15.3±0.4 and 15.2±0.4; baseline (day 0), 12.8±0.9 and 12.6±0.9; day 30, 14.8±0.4 and 15.2±0.5; and day 180, 15.0±0.4 and 15.0±0.4. CDVA was assessed using a Snellen chart at the baseline and/or screening visits, day 30, and day 180. The vast majority of subjects had little or no change in CDVA at days 30 and 180. One subject had a decrease ≥2 lines in the right eye at day 30 and another had an increase ≥2 lines in the right eye at day 30. Both subjects had a CDVA within one line of baseline at day 180.
Slit-lamp biomicroscopy and indirect ophthalmoscopy exams showed no clinically significant changes from baseline at any of the follow-up visits out to day 180. Visual examination of the posterior segment of the eye by indirect ophthalmoscopy with dilation was performed at the screening visit. Slit-lamp biomicroscopy was performed at each visit and a change in score ≥2 was considered clinically meaningful. There were fewer subjects with a score increase ≥2 than there were subjects with a decrease, or improvement, in score ≥2. The lashes of all subjects (33/33, 100%) were evaluated as normal for both eyes. Other ocular surface findings observed during slit-lamp examinations included a decrease in conjunctival edema and erythema and a decrease in tear film debris.
Nasal endoscopies of all subjects at baseline and at study exit were conducted by an otolaryngologist and showed no changes between baseline and study exit, with the exception of one subject who had a lesion in the fossa of the left nasopharynx. The lesion, which was diagnosed as reactive lymphoid tissue, was found to be benign upon biopsy and deemed to be unrelated to the study device.
Discussion
In this study, the application of gentle electrical stimulation to the anterior ethmoidal nerve inside the nasal cavity led to an increase in tearing, often starting within seconds of stimulation. Schirmer scores measured during intranasal stimulation showed a robust increase relative to the corresponding non-stimulated Schirmer scores at all time points. There was an initial transient period of ~2 weeks, when the increase in tearing stabilized to a level slightly lower than the level observed on day 0. This response was observed across all severity levels, where the average increase in tearing at day 180 was 7.5 mm for patients in DEWS severity category I and II, and 6.8 mm for patients in DEWS category III and IV. These findings show that the nasolacrimal stimulator is effective for induction of near-immediate tearing. These data are consistent with the natural role of the NLR in bolus tearing and its contribution to 35% of basal tearing.19
Patient diary data generally showed an improvement in ocular comfort lasting for ~3 hours beyond the application. Based on the average number of uses per day, it can be deduced that patients used the device every ~6 hours. The duration of comfort may be explained in part by the endogenous nature of the tears, which may consist of mucins, proteins, growth factors, and lipids typically found in tears. A parallel may be drawn to the use of autologous serum tears in the management of dry eye, which are believed to be effective due to the variety of endogenous proteins and other constituents in the serum, similar to natural tears.35,36 Patients generally reported a high degree of overall satisfaction. When surveyed after the study, 83% of patients stated that they would recommend the device to friends or family members with dry eye. The outcome of the secondary clinical endpoint, ocular surface staining, is promising and warrants further investigation in a study utilizing ocular surface staining as an entry criterion. Nearly all patients exhibited a reduction in symptoms as measured by VAS and OSDI; these endpoints also warrant further investigation.
The present investigation showed promising initial results; however, there were some limitations. This was a single-arm, single-center, open-label study that consisted of an ethnically homogeneous cohort. Although mean improvements in ocular surface staining were observed, the study was not designed to specifically assess these endpoints. Additionally, the primary focus of the study was the aqueous layer of the tear film, produced predominantly by the lacrimal gland. A further study of the possible effect of intranasal stimulation on other team film layers is also warranted. Furthermore, TBUT was measured prior to nasal stimulation and merits further investigation. Finally, the study device itself was a prototype version of the Oculeve device and lacked enhancements found in subsequent versions that may further enhance the patient experience, overall satisfaction, and clinical effect.
Conclusion
The present strategy of stimulating sensory nerves inside the nasal cavity may offer a new paradigm for enhancing tear production in DED. Many patients showed significant improvements in chronic signs and symptoms. The robust increase in natural tearing was demonstrated across a range of DED severities (DEWS levels I–IV), and it is possible that combining an anti-inflammatory agent with intranasal electrical stimulation (which was the case for 10% of the patients who were taking topical cyclosporine in the current study) could lead to even better outcomes.
Acknowledgments
Funding for this study was provided by Oculeve, Inc. (South San Francisco, CA, USA), now wholly owned by Allergan, Plc (Dublin, Ireland). Assistance in the preparation of the manuscript was provided by Aron D Ross, PhD, Triton Biomedical, Inc. (Laguna Beach, CA, USA) and by Manfred Franke, PhD, Oculeve, Inc.
Disclosure
NJF is a consultant to and has equity in Oculeve and serves on the Speaker’s Bureau of Allergan, Inc. JL is an employee and stockholder of Allergan, Inc. SNB is an employee of Allergan, Inc. AC has served as a consultant to Oculeve, Inc. The authors report no other conflicts of interest in this work.
References
The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):75–92. | ||
Behrens A, Doyle JJ, Stern L, et al; Dysfunctional Tear Syndrome Study Group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907. | ||
Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31(3):271–285. | ||
O’Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004;4:314–319. | ||
Stapleton F, Garrett Q, Chan C, Craig JP. The epidemiology of dry eye disease. In: Chan C, editor. Dry Eye: A Practical Approach. Springer, Berlin; 2015:21–29. | ||
Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. | ||
Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. | ||
Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. | ||
Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients’ lives: comparisons to a US normative sample. Invest Ophthalmol Vis Sci. 2005;46(1):46–50. | ||
Schiffman RM, Walt JG, Jacobson G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmol. 2003;110(7):1412–1419. | ||
Dalzell MD. Dry eye: prevalence, utilization, and economic implications. Manag Care. 2003;12(12 suppl):9–13. | ||
American Academy of Ophthalmology. Preferred Practice Pattern®: Dry Eye Syndrome. 2013. Available at: http://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp--2013. Accessed Nov 30, 2015. | ||
Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013;11(4):246–258. | ||
Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–177. | ||
Rocha EM, Alves M, Rios JD, Dartt DA. The aging lacrimal gland: changes in structure and function. Ocul Surf. 2008;6(4):162–174. | ||
Foster CS, Ekong AS, Anzaar F, et al. Dry Eye Syndrome. Medscape Reference; 2011. WebMD, LLC; NY, New York. | ||
Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. | ||
Kossler AL, Wang J, Feuer W, Tse DT. Neurostimulation of the lacrimal nerve for enhanced tear production. Ophthal Plast Reconstr Surg. 2015;31(2):145–151. | ||
Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16(6):645–648. | ||
van Setten G. Present and future perspectives in the treatment of dry eye disease. Eur Ophthal Rev. 2014;8(2):91–92. | ||
Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens. 2010;36(1):33–38. | ||
Gulati A, Dana R. Keratocjunctivitis sicca: clinical aspects. In: Smolin G, Foster CS, Azar DT, Dohlman CH, editors. Smolin and Thoft’s The Cornea: Scientific Foundations and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins; 2005:603–628. | ||
Tong L, Petznick A, Lee S, Tan J. Choice of artificial tear formulation for patients with dry eye: where do we start? Cornea. 2012;31(suppl 1):S32–S36. | ||
Mazow ML, McCall T, Prager TC. Lodged intracanalicular plugs as a cause of lacrimal obstruction. Ophthal Plast Reconstr Surg. 2007;23(2):138–142. | ||
Sakamoto A, Kitagawa K, Tatami A. Efficacy and retention rate of two types of silicone punctal plugs in patients with and without Sjögren syndrome. Cornea. 2004;23(3):249–254. | ||
Horwath-Winter J, Thaci A, Gruber A, Boldin I. Long-term retention rates and complications of silicone punctal plugs in dry eye. Am J Ophthalmol. 2007;144(3):441–444. | ||
Sugita J, Yokoi N, Fullwood NJ, et al. The detection of bacteria and bacterial biofilms in punctal plug holes. Cornea. 2001;20(4):362–365. | ||
Johnson MD, Lim HH, Netoff TI, et al. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. 2013;60(3):610–624. | ||
Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature. 2013;496(7444):159–161. | ||
Gofeld M. New horizons in neuromodulation. Curr Pain Headache Rep. 2014;18(3):397. | ||
Goroszeniuk and Pang. Peripheral neuromodulation: a review. Curr Pain Headache Rep. 2014;18(5):412. | ||
Kacker R, Lay A, Das A. Electrical and mechanical office-based neuromodulation. Urol Clin North Am. 2013;40(4):581–589. | ||
Luan S, Williams I, Nikolic K, Constandinou TG. Neuromodulation: present and emerging methods. Front Neuroeng. 2014;7:27. | ||
Agnesi F, Johnson MD, Vitek JL. Deep brain stimulation: how does it work? Handb Clin Neurol. 2013;116:39–54. | ||
Poon AC, Geerling G, Dart JK, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85(10):1188–1197. | ||
Singh R, Gangwar A, Singh S, Sharma BD. Autologous serum in treatment of dry eye disorder: an evaluation. J Appl Pharm Sci. 2012;2(6):159–163. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.