Back to Journals » International Journal of General Medicine » Volume 15
A Nomogram for Predicting the Risk of Critical Limb Ischemia in Adults with Hypertension: A Retrospective Study
Authors Xu D, Zhu X , Huo J, Xie X, Huang C, Fang X, Yin T
Received 3 June 2022
Accepted for publication 27 October 2022
Published 18 November 2022 Volume 2022:15 Pages 8205—8216
DOI https://doi.org/10.2147/IJGM.S342448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dong Xu,1 Xu Zhu,2 Junyu Huo,2 Xupin Xie,1 Changpin Huang,1 Xin Fang,1 Ting Yin3
1Department of Vascular Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Cardiology, Jiangsu Province Hospital and Nanjing Medical University, First Affiliated Hospital, Nanjing, Jiangsu, People’s Republic of China; 3Intensive Care Unit, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Ting Yin, Intensive Care Unit, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310000, People’s Republic of China, Tel +86 13777879077, Fax +86 56005600, Email [email protected] Xin Fang, Department of Vascular, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310000, People’s Republic of China, Tel +86 13867478324, Fax +86 56005600, Email [email protected]
Purpose: Peripheral arterial disease (PAD) presenting with underlying hypertension (HTN) poses a higher risk of bilateral lower limb amputation than PAD patients without HTN. While the role of HTN management of PAD patients has received limited attention. We analyzed the clinical characteristics of PAD in adults with HTN and explored risk factors for PAD to construct a nomogram for evaluating critical limb ischemia (CLI) and lesion severity.
Methods Patients and Methods: Between January 2014 and December 2019, we retrospectively evaluated 1886 patients with peripheral artery disease with coexisting HTN. Patients were randomly divided into training (n = 1320, 70%) and validation cohorts (n = 566, 30%), and according to the subjective experience of PAD [Fontaine classification (I–II vs III–IV)], patients were further classified into intermittent claudication (IC) and CLI groups. LASSO regression and multivariate Cox proportional hazard analyses were used to construct a nomogram using variables defined in the training cohort, which was validated in the validation cohort. The evaluation of the predictive discriminative, accuracy and clinical application are further analyzed.
Results: In the training cohort, optimal independent factors included age, male sex, body mass index, diabetes mellitus, heart rate, triglyceride, and uric acid (AM-BDHTU), which were included in the nomogram predicting the CLI risk (all P < 0.05). The C-index values for CLI risk in PAD with HTN patients were 0.729 (95% CI: 0.704– 0.807) and 0.728 (95% CI: 0.652– 0.744) in the training and validation sets, respectively. Calibration curves indicated good consistency between predicted and actual outcomes. DCA confirmed the clinical utility of the diagnostic model.
Conclusion: The AM-BDHTU nomogram, constructed and validated using simple to obtain clinical variables, when combined with the Fontaine classification, effectively predicts the risk of CLI among PAD patients with HTN.
Keywords: peripheral arterial disease, hypertension, vascular-related complications, LASSO regression, nomogram, predictive diagnosis
Introduction
Peripheral arterial disease (PAD) is a form of atherosclerosis (AS) that causes a high incidence of lower extremity adverse events and nontraumatic amputations and affects more than 200 million people worldwide.1–3 PAD is a degenerative disease that often occurs in senior adults, a population also presenting the concomitant risks for major cardiovascular events and higher mortality.4 Management of PAD requires a strict control of cardiovascular risk factors, including hypertension (HTN), diabetes mellitus (DM), and dyslipidemia. Effective attention has been paid to the importance of managing glucose and lipid levels.5 However, the role of HTN in the management of PAD patients has received limited attention.
Epidemiological studies have also shown that the prevalence of systolic HTN6 and aortic stiffness7 among patients with PAD is also increasing. HTN is an independent risk factor for progressive AS, which is the underlying pathophysiological cause of PAD, and an adjusted odds ratio (ORs) of 1.25 for each increase in 10-mmHg systolic blood pressure has been reported for the development of PAD.8 Thus, the combination of these two factors leads to more severe vascular disease. The combination of atherosclerotic effects on the blood vessel framework of the lower limbs and HTN has resulted in higher mortality rates in both developed and developing countries worldwide.9 Recent research reported that treatment with HTN using calcium channel blockers (CCB) was strongly associated with a decrease in the PAD.49 And contribution of the angiotensin II (Ang II) pathway and an overactive sympathetic nervous system (SNS) can accelerate atherogenesis, but may represent other target of HTN and PAD treatment.10–12 Although sharing a fairly common pathophysiology, risk factors, and management, the presentation of symptoms in most patients with PAD and HTN is often obscure and PAD is difficult to diagnose at an early stage.13 AS usually develops gradually and silently over time, and its associated complications are markedly greater in patients with HTN.14,15 In particular, critical limb ischemia (CLI), an adverse effect that is commonly observed in combination with existing HTN, is associated with a poor prognosis of higher 5-year mortality.16 Chronic limb-threatening ischemia (CLTI) is associated with mortality, amputation, and poor quality of life. Thus, the identification of predictive clinical signals and stratification of disease risk is urgently needed to help markedly reduce the progression of the adverse macroangiopathy.
PAD itself can be considered a marker for systemic atherosclerotic processes,15 HTN are likely to be one of the main driving forces.44 PAD and its ABI index was positively and independently associated with the yearly change in SBP and hypertension incidence.45,46 The migration of smooth muscle cells, the reduction of elastic fibers, and aggregation of macrophages and T lymphocytes at the arterial wall all contribute to endothelial dysfunction, AS formation, and calcification of the atherosclerotic plaque, which predisposes to vascular injury and the appearance and development of PAD or HTN.14,17 However, limited data regarding the clinical characteristics of patients with PAD with co-existing HTN are available. In particular, subjective symptoms are the main motivation for patients to seek care.18 The international criteria for the standard diagnosis of PAD, the Fontaine classification, emphasize the diagnostic importance of symptoms, and when combined with clinical characteristics of HTN these are essential to accurately manage patients with coexisting PAD and HTN, and allow to start an appropriate treatment regimen to avoid severe vascular events. Given the common underlying pathology of PAD and HTN, it is important to define the characteristics of these patients to assess the risk for serious life-threatening vascular-related complications.19
Early identification and effective intervention are indispensable for clinical decision-making for patients with PAD and HTN. The nomogram, a graphical calculation tool designed to allow prediction of the overall probability of disease progression in combination with the current clinical characteristics of an individual patient, can provide clinicians with an accurate estimate of risk for different diseases.20 Thus, the establishment of a nomogram model could help in the early detection of disease progression and identify high-risk cases, and further promote initial management to help reduce significant complications and mortality.
In this study, we established a novel AM-BDHTU nomogram integrating the Fontaine classification and clinicopathological characteristics to establish easy-to-use and efficient diagnostic tool to predict the probability of risk of Fontaine I–II stage (intermittent claudication [IC]) progression to stage III–IV (critical limb ischemia [CLI]) disease in subjects with PAD and HTN.
Methods and Study Population
Study Design and Patient Selection
This was a single-center, retrospective, observational study. Patients with HTN and PAD hospitalized for treatment at the Affiliated Hangzhou First People’s Hospital of Zhejiang University School of Medicine (Zhejiang, China) between January 2014 and December 2019 were enrolled. Ethical approval for the study was obtained from the Ethics Committee and Institutional Review Board of the Affiliated Hangzhou First People’s Hospital. Study procedures were conducted in accordance with the Declaration of Helsinki.
Baseline data (including sex, age, body mass index [BMI], smoking habits, alcohol use, blood pressure, duration of CLI), laboratory results for tests conducted at hospital laboratory after admission (complete blood count, biochemical tests, and coagulation function) were collected.
Fontaine stage (stage I: defined as asymptomatic or effort pain; stage IIA/IIB: pain starting over a walking distance of >200 meter / < 200 meter; stage III: pain in rest; stage IV: trophic lesions in the lower extremities) was determined standardized report of possible walking distance supervised by a senior vascular surgeon.47,48 The degree of IC and CLI was established according to Fontaine stage (I–II vs III–IV) with symptoms and signs of chronic PAD including lower extremity numbness, chills, fear of cold, intermittent claudication, rest pain and gangrene, distal arterial pulse weakened or absent.21,30 Patients with PAD and HTN (defined as diastolic blood pressure [DBP] ≥90 mmHg or systolic blood pressure [SBP] ≥140 mmHg or taking antihypertensive medicine) were defined according to the corresponding guidelines.14,22
The exclusion criteria were as follows: age <18 years old; arteritis and other systemic diseases causing arterial embolization; severe liver and kidney dysfunction; malnutrition; malignant tumors; malignant anemia; history of myocardial infarction or stroke or cerebrovascular incidents within 1 month of the study; acute inflammatory disease; pregnant females; lactating mothers; and incomplete follow-up data.
In total, 1886 patients aged above 18 years were enrolled in the study and were assessed for eligibility from January 2014 to December 2019. Patients withdrawn informed consent (N = 7) and blood pressure of patients returns to normal without oral anti-HTN medicine (N = 13) excluded of the research. Among, 1886 patients divided training cohort (N = 1320, 70%) and validation cohort (N = 566, 30%) were enrolled for the further analysis (Shown in Figure S1). The analyses were stratified by Fontaine stages (according to the corresponding ICD-10 code) during the index stage: stage I–II for the IC group vs stage III/IV for the CLI group.15,23
Statistical Analysis
The Kolmogorov–Smirnov statistical test was used to check the normality of continuous variables. Continuous data and categorical data were expressed as mean ± standard or median (interquartile range), and categorical characteristics were expressed as n (%). Baseline variables were compared using the unpaired t-test (normal distribution) or the Mann–Whitney U-test (non-normal distribution) for continuous variables, and Pearson’s chi-square test for categorical variables.
The Least absolute shrinkage and selection operator (LASSO) regression analysis was used for data dimension and predictor selection. Regarding LASSO regression, we used a 10-fold cross-validation method to identify the optimal lambda of regularization parameter and the area under the curve (AUC) values for data dimension reduction and feature selection in the training cohort. Multivariate logistic regression analysis based on LASSO regression results was used to develop a predictive model and a nomogram for PAD patients with coexisting HTN.24 The discriminatory capacity of the model was determined by calculating the AUC. The consistency of the predicted and actual results was evaluated using the Hosmer–Lemeshow (H-L) χ2 test and plotting the calibration curve. Decision curve analysis (DCA)25 and clinical impact curve (CIC) analysis were applied to assess clinical utility of the diagnostic model.26 Statistical analyses were performed using SPSS version 24 (IBM Corporation, Armonk, NY, USA) and R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria) including the functions “glmnet”, “ggplot2”, “pROC”, “rms”, “ResourceSelection”, and “rmda”. All tests were two-sided and P <0.05 was considered statistically significant.
Results
Baseline Characteristics in the Training and Validation Cohorts
A total of 1886 patients (males: 66.33%, n = 1251; females: 33.67%, n = 635; aged, 64.68±8.93 years old) were randomly allocated to the training cohort (n = 1321, 70%) or the validation cohort (n = 566, 30%). According to Fontaine stage, patients were further divided into the IC group (Fontaine I–II) (58.7% males) and the CLI group (Fontaine III–IV) (55.6% males). The demographics and clinical characteristics of both cohorts are summarized in Table 1.
 |
Table 1 Baseline Characteristics of Clinical Data |
High-risk groups were more likely to include older adults, males, smokers, and alcohol users, adults with a higher BMI, and individuals presenting DM comorbidities, lower estimated glomerular filtration rate (eGFR), lower triglyceride (TG) levels, and lower hemoglobin and platelet count (PLT) compared to individuals in the low-risk group (P < 0.05). The characteristics of the corresponding training and validation sets were comparable.
Multivariable Selection for Prediction Model
We performed a LASSO regression analysis in the training cohort and seven variables were identified: age, sex, uric acid (UA) level, TG, mean heart rate (MHR), BMI, and DM (Figure 1). The results of multivariate logistic regression according to the variables selected by the LASSO regression method were used to build the model adjusted for the seven variables indicated above (Table 2). The optimal model (AM-BDHTU) revealed that the following seven variables were independently associated with PAD patients with HTN: older age (odds ratio [OR], 1.04; 95% CI: 1.03–1.06); male sex (OR, 2.14; 95% CI: 1.61–2.87) and Log transformed UA (OR, 3.46; 95% CI: 1.11–10.84); higher TG (OR, 1.17; 95% CI, 1.06–1.28) and MHR (OR, 1.03; 95% CI, 1.02–1.04); BMI (OR, 1.13; 95% CI, 1.09–1.18), and DM (OR, 1.96; 95% CI, 1.48–2.60) compared to those with HTN complications.
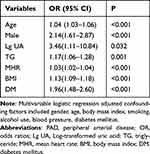 |
Table 2 Odds Ratios of the Multivariable Logistic Regression Model Predicting PAD |
Nomogram Development
To develop an AM-BDHTU score system for identifying patients with high-risk PAD with concomitant HTN, a multivariate LASSO regression analysis was performed using the training set to detect independent diagnostic factors (Supplementary file Table 1). The nomogram was constructed accordingly to predict the risk of LI in patients with PAD and HTN and was used to define patients at high-risk stages (Figure 2).
Model Evaluation
Model performance was assessed using the validation set. The AUROCs indicated the discriminative ability of the AM-BDHTU nomogram in the training and validation cohorts were 0.729 and 0.728, respectively. There was no significant difference between the two cohorts (P = 0.994), suggesting a remarkable sensitivity and specificity of the nomogram in the clinical context (Figure 3). The AM-BDHTU nomogram model predicted the probability of LI in PAD (Fontaine IIB–IV) patients with HTN. The calibration curve showed that the nomogram exhibited favorable concordance between actual results and predicted probabilities (Hosmer–Lemeshow test P = 0.664) (Figure 4). The DCA in the training cohort was performed to assess the net benefit of the clinical risk prediction model. The threshold probability of PAD and co-existing HTN was 54–85%. The application of this nomogram would add a significant benefit to the neglected management of these factors and in improving the inadequate intervention scheme (Figure 5).
Optimal Model Analysis
The clinical applicability of the risk prediction nomogram was evaluated using CIC analysis and showed that the “high risk number” curve was close to the “high risk number with the events” curve at a high risk threshold of 0.65 to 1 (Figure 6). CIC intuitively showed that the nomogram provided a superior overall net benefit based on the practical ranges of threshold probabilities and impacted on patient outcomes, which indicated that the model possessed significant predictive value of identifying high-risk PAD cases among HTN patients.
Discussion
In the study, we explored the application of a nomogram to predict the development of high-risk PAD among patients with HTN. An increasing number of patients with PAD who present with HTN comorbidities seek medical treatment in hospitals. Our AM-BDHTU nomogram incorporated variables, including age, sex, BMI, UA levels, TG levels, the MHR, and the presence of DM, and showed good discriminatory ability, calibration, and value in clinical application. Combined with these clinical characteristics and the Fontaine stage, this nomogram used as a prognostic indicator would be beneficial for early assessment and high-risk stratification of identification of PAD patients with HTN at high-risk of CLI.
In recent decades, research on vascular-related diseases has grown explosively due to changing lifestyle and aging of the population. PAD has been recognized as a characteristic pathological disease of systemic atherosclerosis, posing a public health crisis and a threat to health safety.13,26 In particular, HTN leads to approximately 7.6 million premature deaths each year (≈13.5% of the global total).27 HTN can alter the release of endothelial-derived vasoactive factors and adverse effects of systemic arterial vessels, contributing to arterial disease of the lower extremities.28 In fact, very few patients with PAD were not associated with other cardiovascular risk factors. Therefore, the screening of such high-risk patients for critical LI should always be considered when assessing an PAD patient presenting with HTN in clinical practice. The Fontaine classification is widely used in the clinical evaluation of PAD and could also be applied in the planning of the treatment of PAD.29 However, a previous study evaluating the Fontaine classification showed that the ratio of chronic vascular-related diseases such as HTN among patients with ischemic limbs was relatively low.30 The advantage of this nomogram scoring system combined with Fontaine diagnostic criteria is that it could simultaneously assess patients with PAD with HTN at the early stages of hospital admission. Meanwhile, clinicians could take applied optional steps for further examination in the identified high-risk patients with PAD, and also reduce the potential for side effects from unnecessary invasive care, thus reducing the burden of medical resources. Although PAD poses a challenge to treatment in general, most patients with and early diagnosis of PAD can be controlled by prompt detection and proper management of risk factors using appropriate regimens. Several risk factors for PAD have been identified, such as aging, smoking, coexisting diabetes, and other ischemic comorbidities.1,4,16 Most studies have highlighted DM-associated macrovascular and microvascular injury of the lower extremities.4,31 Few studies have described PAD risk prediction models in patients with HTN.
Nomograms, which can easily estimate the predicted and diagnosis probability of different predictors alone or in combination, have been widely used as a clinical tool in chronic disease and oncology. The scoring standard is formulated based on the size of the regression coefficients of all independent variables. Thus, the nomogram allows to visualize a predictive model. Further, a nomogram is more advantageous than a traditional diagnostic criterion, like Fontaine or Rutherford mainly emphasized subjective symptoms, as it can provide personalized predictive diagnosis and treatment, and can quickly guide clinical practice. LASSO regression optimization variance collinearity is a good solution for evaluating vascular-related diseases associated with other chronic pathological mechanisms. The present study was the first to explore a diagnostic prediction model of CLI in patients with PAD presenting with HTN. The seven ease of acquisition variables (age, male, BMI, UA, TG, MHR, DM) included in the nomogram were identified by LASSO regression analysis, which is considered to be effective for selecting predictors by univariate analysis. It is especially suitable for primary hospitals that cannot carry out imaging examination or chronic disease management clinics.
Aging, male sex, and DM32 were independent factors associated with adverse cardiovascular events and vascular damage, also confirmed by our nomogram scoring system. The mean age of our subjects was younger than that of patients exhibiting only PAD in clinical studies, which indicates that patients with coexisting PAD and HTN are more susceptible to pathological changes in the vasculature. Our results also demonstrated that a higher resting heart rate (HR) is associated with a higher risk of PAD among patients with HTN. Similar results were observed in patients with atherosclerotic peripheral artery disease.33 Increased resting HR decreases in arterial distensibility and arterial stiffness,34 which induce the first detectable manifestations of adverse structural and functional changes within the vessel wall.35 In addition, an increased HR gives rise to high arterial pulsatile flow and shear stress promotes atherosclerotic lesion formation, which is marked in patients with HTN.36 HR is independently correlated with arteriolar lesion sclerosis and cohort studies have shown that hypertensive patients with a high resting HR have an increased risk of all-cause and cardiovascular death.37,38 Apart from age, MHR was the second most important factor in our prediction model. A possible explanation for our optimized model was that influencing factors associated with high blood pressure also led to pathological manifestations. One explanation for this finding is that higher risk of lower limb ischemia and amputation in PAD patients with concomitant HTN.
Earlier detection of vascular damage and screening for potential clinical events by other atherosclerotic biomarkers in the plasma was expected. The BMI level was slightly higher than the normal range in the population, while increased TG levels were possibly due to a decrease in lipoprotein lipase activity and an inhibition of reverse cholesterol transport.39 Furthermore, overweight and obese status induced glucose and lipid metabolism disorders that participate to the endocrine regulation of the vascular system. Visceral adipose tissue, excessive abdominal fat, and its concomitant metabolic characteristics are important key mediators that influence blood pressure, lipid profiles, and inflammatory status,39,40 as well as generate atrial endothelium dysfunction. UA is also associated with known risk factors for the progression of kidney disease, including HTN41 and atherosclerosis.42 The prognosis of cardiovascular diseases has been associated with a decrease in renal reperfusion and eGFR.43 In fact, the variables in our study yielded an estimated risk assessment based on factors that may be potentially modified by preventative measures for PAD and HTN. Further, these continuous outcomes instead of one cutoff value could provide a more visualized probabilistic risk assessment.
The AM-BDHTU showed good discriminatory ability and calibration, and DCA and CIC analyses showed its clinical significance and utility. These seven predictors identified in this study are easily acquired in PAD patients with HTN, providing an early-to-use diagnostic tool and allowing a supplementary evaluation in accordance with the Fontaine classification to achieve prompt clinical intervention. This simple and cost-free scoring system may be helpful in continued screening of patients at high-risk of CLI.
To our knowledge, few studies have explored the utility of a risk prediction tool for high-risk PAD patients with HTN to identify the probability of CLI. Nonetheless, our findings should be considered in light of several limitations. First, the study only used hospital data to ensure that complete clinical variables were collected, which could have caused a specific selection bias due to the lack of inclusion of outpatient and primary care settings. Second, the nomogram was built based on a 5-year retrospective cohort study conducted in China and was conducted using internal validation. Therefore, whether the nomogram is generalizable to other populations requires further verification in multicenter studies. Third, the data relative to the more noninvasive and invasive imaging evaluation of ischemia were not included in our analysis, as these data were not available in the original dataset. Expanding the study population and stratified analysis of cardiovascular complications should be considered to improve popularization meanings of the model in the future. However, it is important to point out that 1884 subjects were identified in our cohort and completed functional performance measures with moderate statistical power.
In conclusion, the current study constructed an easy-going AM-BDHTU score nomogram that plays a convincing role in the evaluation of the risk of progression of the CLI risk in PAD patients concomitant HTN. This nomogram could complement the diagnostic identification of high-risk PAD patients presenting underlying HTN, especially in patients with vascular-related risk factors. Nonetheless, further study with larger sample sizes and multiple centers are essential to verify our conclusions.
In conclusion, the current study constructed an easy-going AM-BDHTU score nomogram could complement the diagnostic identification of high-risk PAD patients presenting underlying HTN, especially in patients with vascular-related risk factors. Nonetheless, further study with larger sample sizes and multiple centers are essential to verify our conclusions.
Ethics Statement
The studies involving human participants were reviewed and approved by Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (Zhejiang, China) ethics committee. The patients provided their written informed consent to participate in this study.
Acknowledgments
We gratefully acknowledge our patients in this study and their families for their consent and participation in this study. Ting Yin and Xin Fang are joint corresponding authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet. 2013;382:1312–1314. doi:10.1016/S0140-6736(13)61576-7
2. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):1465–1508. doi:10.1016/j.jacc.2016.11.008
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2013;127(1):e6–e245.
4. Nativel M, Potier L, Alexandre L, et al. Lower extremity arterial disease in patients with diabetes: a contemporary narrative review. Cardiovasc Diabetol. 2018;17:138.
5. The Japanese Society For Vascular Surgery Jclimb Committee, Ncd Jclimb Analytical Team. 2018 Japan Critical Limb Ischemia Database (JCLIMB) annual report. Ann Vasc Dis. 2021;14(2):202–230.
6. Safar ME, Laurent S, Asmar RE, Safavian A, London GM. Systolic hypertension in patients with arteriosclerosis obliterans of the lower limbs. Angiology. 1987;38(4):287–295.
7. Catalano M, Scandale G. Increased aortic stiffness and related factors in patients with peripheral arterial disease. J Clin Hypertens. 2013;15(10):712–716.
8. Tanaka Y. Arteriosclerosis obliterans (ASO) in diabetic patients. Nihon Rinsho. 2003;61(7):1187–1193.
9. Gkaliagkousi E, Douma S. The pathogenesis of arterial stiffness and its prognostic value in essential hypertension and cardiovascular diseases. Hippokratia. 2009;13:70–75.
10. MazzolaiL, Duchosal MA, Korber M, et al. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE−/− mice. Hypertension. 2004;44(3):277–282. doi:10.1161/01.HYP.0000140269.55873.7b
11. Zubcevic J, Jun JY, Kim S, et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63(3):542–550. doi:10.1161/HYPERTENSIONAHA.113.02722
12. Santisteban MM, Ahmari N, Carvajal JM, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117(2):178–191. doi:10.1161/CIRCRESAHA.117.305853
13. Marie D, Heather L, Neal R, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. Circulation. 2017;135(12):e726–e779. doi:10.1161/CIR.0000000000000471
14. Sasamura H, Itoh H. Hypertension and arteriosclerosis. Nihon Rinsho. 2011;69(1):125–130.
15. Bartholomew JR, Olin JW. Pathophysiology of peripheral arterial disease and risk factors for its development. Clevel Clin J Med. 2006;73(Suppl 4):S8–S14. doi:10.3949/ccjm.73.Suppl_4.S8
16. McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116(9):1540–1550. doi:10.1161/CIRCRESAHA.114.303517
17. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi:10.1056/NEJMra043430
18. Brunner EJ, Shipley MJ, Witte DR, et al. Arterial stiffness, physical function and functional limitation: the Whitehall II study. Hypertension. 2011;57(5):1003–1009. doi:10.1161/HYPERTENSIONAHA.110.168864
19. Abaraogu UO, Ezenwankwo EF, Dall PM, Seenan CA. Living a burdensome and demanding life: a qualitative systematic review of the patients experiences of peripheral arterial disease. PLoS One. 2018;13(11):e0207456. doi:10.1371/journal.pone.0207456
20. Rose PG, Java J, Whitney CW, et al. Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/gynecologic oncology group randomized trials of chemoradiotherapy. J Clin Oncol. 2015;33(19):2136–2142. doi:10.1200/JCO.2014.57.7122
21. Tendera M, Aboyans V, Ml B, et al.; European Stroke Organisation. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(22):2851–2906. doi:10.1093/eurheartj/ehr211
22. Oparil S, Acelajado MC, Bakris GL, et al. Hypertension. Nat Rev Dis Primers. 2018;4:18014. doi:10.1038/nrdp.2018.14
23. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–S75. doi:10.1016/j.ejvs.2006.09.024
24. Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B. 1996;58:267–288.
25. Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. doi:10.1016/j.eururo.2018.08.038
26. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58:S1–S109. doi:10.1016/j.ejvs.2019.05.006
27. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. International society of hypertension. Lancet. 2008;371(9623):1513–1518. doi:10.1016/S0140-6736(08)60655-8
28. Wilson C, Zhang X, Buckley C, Helen R, Matthew D, John G. Increased vascular contractility in hypertension results from impaired endothelial calcium signaling. Hypertension. 2019;74(5):1200–1214. doi:10.1161/HYPERTENSIONAHA.119.13791
29. Azuma N. The diagnostic classification of critical limb ischemia. Ann Vasc Dis. 2018;11(4):449–457. doi:10.3400/avd.ra.18-00122
30. Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21:499–533.
31. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80.
32. Mita T, Katakami N, Okada Y, et al. Protocol of a prospective observational study on the relationship between glucose fluctuation and cardiovascular events in patients with type 2 diabetes. Diabetes Ther. 2019;10(5):1565–1575.
33. Iwamoto A, Kajikawa M, Maruhashi T, et al. Vascular function and intima-media thickness of a leg artery in peripheral artery disease: a comparison of Buerger disease and atherosclerotic peripheral artery disease. J Atheroscler Thromb. 2016;23(11):1261–1269.
34. Seamus P, Mouaz H, David A, et al. Association of resting heart rate with carotid and aortic arterial stiffness: Multi-Ethnic Study of Atherosclerosis (Mesa). Hypertension. 2013;62(3). doi:10.1161/HYPERTENSIONAHA.113.01605
35. Mircoli L, Mangoni AA, Giannattasio C, Mancia G, Ferrari AU. Heart rate-dependent stiffening of large arteries in intact and sympathectomized rats. Hypertension. 1999;34:598–602.
36. Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens. 1997;15:3–17.
37. Zhao MX, Zhao Q, Zheng M, et al. Effect of resting heart rate on the risk of all-cause death in Chinese patients with hypertension: analysis of the Kailuan follow-up study. BMJ Open. 2020;10(3):e032699.
38. Kouvas N, Tsioufis C, Vogiatzakis N, et al. Heart rate and blood pressure: “connecting the dots” in epidemiology and pathophysiology. Angiology. 2018;69(8):660–665.
39. Nakaya K, Ayaori M, Uto-Kondo H, et al. Cilostazol enhances macrophage reverse cholesterol transport in vitro and in vivo. Atherosclerosis. 2010;213(1):135–141.
40. LeBlanc S, Coulombe F, Olivier F, et al. Hypertriglyceridemic waist: a simple marker of high‐risk atherosclerosis features associated with excess visceral adiposity/ectopic fat. J Am Heart Assoc. 2018;7(8):e008139.
41. Camhi SM, Katzmarzyk PT, Broyles ST, et al. Subclinical atherosclerosis and metabolic risk: role of body mass index and waist circumference. Metab Syndr Relat Disord. 2011;9:119–125.
42. Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):98–303.
43. Tavil Y, Kaya MG, Oktar SO, et al. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis. 2008;197(1):159–163.
44. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Mh C. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14(3):156–170.
45. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128(12):1818–1832.
46. Clement DL. Hypertension and peripheral artery disease. J Hypertens. 2020;38(12):2378–2379.
47. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the American Association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation. 2006;113:e463–e654.
48. Fontaine R. Long-term results of restorative arterial surgery in obstructive diseases of the arteries. J Cardiovasc Surg. 1964;5:463–472.
49. Shetty S, Malik AH, Feringa H, El Accaoui R, Girotra S. Meta-analysis evaluating calcium channel blockers and the risk of peripheral arterial disease in patients with hypertension. Am J Cardiol. 2020;125(6):907–915.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






