Back to Journals » Clinical Interventions in Aging » Volume 18
A New Scoring System for Predicting Ventricular Arrhythmia Risk in Patients with Acute Myocardial Infarction
Authors Sun L , Han B, Wang Y, Zhu W, Jiang J, Zou A, Chi B , Mao L, Ji Y, Wang Q , Tang L
Received 27 October 2022
Accepted for publication 13 February 2023
Published 21 February 2023 Volume 2023:18 Pages 283—292
DOI https://doi.org/10.2147/CIA.S395121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Ling Sun,1,* Bing Han,2,* Yu Wang,1,* Wenwu Zhu,2 Jianguang Jiang,1 Ailin Zou,1 Boyu Chi,1,3 Lipeng Mao,1,3 Yuan Ji,1 Qingjie Wang,1 Liming Tang4
1Department of Cardiology, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, 213003, People’s Republic of China; 2Department of Cardiology, Xuzhou Central Hospital, Xuzhou Clinical School of Nanjing Medical University, Xuzhou, Jiangsu, 221009, People’s Republic of China; 3Dalian Medical University, Dalian, Liaoning, 116000, People’s Republic of China; 4Department of Gastrointestinal Disease, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, 213003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liming Tang, Center of Gastrointestinal Disease, Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, 29 Xinglong Alley, Changzhou, Jiangsu, 213003, People’s Republic of China, Email [email protected] Yuan Ji, Department of Cardiology, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, 213003, People’s Republic of China, Email [email protected]
Objective: In this study, a risk score for ventricular arrhythmias (VA) were evaluated for predicting the risk of ventricular arrhythmia (VA) of acute myocardial infarction (AMI) patients.
Methods: Patients with AMI were divided into two sets according to whether VA occurred during hospitalization. Another cohort was enrolled for external validation. The area under the curve (AUC) of receiver operating characteristic (ROC) was calculated to evaluate the accuracy of the model.
Results: A total of 1493 eligible patients with AMI were enrolled as the training set, of whom 70 (4.7%) developed VA during hospitalization. In-hospital mortality was significantly higher in the VA set than in the non-VA set (31.4% vs 2.7%, P=0.001). The independent predictors of VA in patients with AMI including Killip grade ≥ 3, STEMI patients, LVEF < 50%, frequent premature ventricular beats, serum potassium < 3.5 mmol/L, type 2 diabetes, and creatinine level. The AUC of the model for predicting VT/VF in the training set was 0.815 (95% CI: 0.763– 0.866). A total of 1149 cases were enrolled from Xuzhou Center Hospital as the external validation set. The AUC of the model in the external validation set for predicting VT/VF was 0.755 (95% CI: 0.687– 0.823). Calibration curves indicated a good consistency between the predicted and the observed probabilities of VA in both sets.
Conclusion: We have established a clinical prediction risk score for predicting the occurrence of VA in AMI patients. The prediction score is easy to use, performs well and can be used to guide clinical practice.
Keywords: ventricular tachycardia, ventricular flutter and fibrillation, risk stratification, scoring system, acute myocardial infarction
A Letter to the Editor has been published for this article.
Introduction
Malignant ventricular arrhythmia (VA) is mainly manifested as persistent ventricular tachycardia (VT), ventricular flutter and fibrillation (VF) and other serious life-threatening arrhythmias originating from the ventricle.1,2 Malignant VA is one of the most common cause of death in patients with acute myocardial infarction (AMI).3,4 Early revascularization can significantly improve the prognosis of AMI patients.5,6 However, VA may still occur due to reperfusion myocardial injury or no-reflow.7–9 Thus, early assessment of VA risk in AMI patients can help clinicians to take active prevention and treatment measures, thus reducing the risk of in-hospital death and improving the prognosis.4,10–12 In this study, we aimed to explore independent predictors of VA in AMI patients during hospitalization, and use them to develop a clinical prediction model.
Methods
Study Population and Grouping
The study population was selected from Changzhou No.2 People’s Hospital (Changzhou Acute Myocardial Infarction Registry dataset, http://www.chictr.org.cn/searchproj.aspx, ChiCTR1800014583). The validation set was selected from Xuzhou Center Hospital (Figure 1).
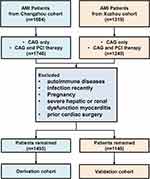 |
Figure 1 Workflow of the inclusion and exclusion of the study subjects. Abbreviations: AMI, acute myocardial infarction; CAG, coronary angiography; PCI, percutaneous coronary intervention. |
Patients diagnosed with AMI in the department of cardiology from two centers from December 2012 to January 2018 were selected and retrospectively analyzed. AMI was diagnosed according to the third edition of Global Definition Criteria for Acute Myocardial Infarction. Inclusion criteria: age >18 years and diagnosis of acute myocardial infarction at admission. Coronary angiography (CAG) was performed among all enrolled patients. Percutaneous coronary intervention (PCI) therapy was then performed following CAG in eligible patients. Patients who underwent coronary artery bypass graft (CABG) after coronary angiography were excluded.
The enrolled patients were divided into two sets according to whether VA occurred during hospitalization: VA set and non-VA set. Exclusion criteria included autoimmune diseases, recent infection, pregnancy, severe hepatic or renal dysfunction, myocarditis, and prior cardiac surgery.
Ethnics Approval
All the enrolled patients had signed informed consent at the time of inclusion, and the project had been approved by the Ethics Committee of Changzhou No.2 People’s Hospital (Ethics number: [2018]KY005-01) and Xuzhou Center Hospital (Ethics number: XZXY-LK-20211021-037). The study was performed in accordance with the Declaration of Helsinki.
Data Collection
The data about patients’ basic characteristics and VA were collected by reviewing the electronic medical record system. The collected variables including the basic information such as age, gender, medical history, diagnosis, vital signs during hospitalization, hospital electrocardiogram characteristics, laboratory parameters, interventional treatment related variables and medications. Echocardiography data during hospitalization were also collected.
Endpoint
The primary endpoint was the occurrence of malignant VA during hospitalization, including persistent ventricular tachycardia, ventricular flutter, and ventricular fibrillation, and the secondary endpoint was the all-cause mortality during hospitalization.
Statistical Analysis
Continuous variables were described using mean ± standard deviation or median, and 25th and 75th percentiles. Categorical variables were expressed by absolute values (percentages, %). Comparisons between two sets were performed using Student’s t-test for continuous variables and chi-square or Fisher exact test for categorical variables. A multivariate logistic regression analysis was performed to sift the independent predictors of VA. A clinical prediction model was constructed. The risk score was calculated by coefficients of the logistic regression model (β value × 10). The area under the curve (AUC) of receiver operating characteristic (ROC) was used to evaluate the accuracy of the prediction model in the training and validation sets. A two-sided test was used. P < 0.05 was considered statistically. SPSS 22.0 was used for statistical analysis.
Results
Baseline Clinical Data
A total of 1493 eligible AMI patients from Changzhou No.2 People’s Hospital were enrolled, of which 70 patients (4.69%) developed VA during hospitalization, and 61 patients (4.09%) died in the hospital. As shown in Table 1, the VA set contained a lower percentage of smokers, higher percentages of patients complicated with diabetes, with Killip grade ≥3, with left ventricular ejection fraction (LVEF) >50%, and with ST-segment elevation myocardial infarction (STEMI). The mortality in VA set was also significantly higher in the VA set (Table 1).
 |
Table 1 Baseline Characteristics of the Patients Included in the Study |
The VA set showed a higher average heart rate, a lower percentage of patients with sinus rhythm, a higher percentage of patients with frequent premature ventricular contractions and QRS wave broadening on baseline electrocardiogram (Table 1). The VA set presented lower serum hemoglobin and potassium levels, but high creatinine and blood glucose levels at admission (Table 1).
Comparison of Treatment-Related Variables Between the Two Sets
Fewer patients in the VA set presented with the left circumflex artery (LCX) as the culprit vessel. A lower percentage of patients received ticagrelor, and a higher percentage of patients received oral or intravenous diuretics in the VA set (Table 2).
 |
Table 2 Medications Treatments and Angiographic Characteristics |
Predictors of Malignant VA
Both univariate and multivariate Logistic regression analyses were used to analyze predictors of malignant VA in patients with AMI. After adjustment, the independent predictors of VA included Killip grade ≥3, STEMI patients, LVEF <50%, frequent premature ventricular beats, serum potassium <3.5 mmol/L, type 2 diabetes, and creatinine level. The risk of VA was scored according to the β value of each variable. The clinical prediction model was established according to the predictors in Table 3.
 |
Table 3 Multivariate Regression Analysis of Predictors of VT/VF Risk in Patients with AMI |
Internal Validation of the Model
Based on the factors in the regression analyses (Table 3), the clinical prediction model was constructed (Figure 2). Its AUC value for predicting VA in the training set was 0.815 (95% CI: 0.763–0.866), suggesting that the model could accurately predict the VA risk in AMI patients Figure 3A.
 |
Figure 3 The ROC curve of the prediction model from two centers. (A) The ROC curve of the prediction model from Changzhou cohort. (B) The ROC curve of the prediction model from Xuzhou cohort. |
External Validation of the Model
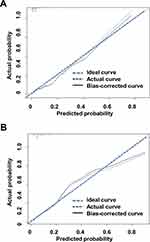
We also validated the accuracy of the model in an external set of 1149 cases from Xuzhou Center Hospital. Baseline and clinical data of the validation set are shown in Table 4. The AUC of the model for predicting VA in the validation set was 0.755 (95% CI: 0.687–0.823) (Figure 3B). Finally, calibration curves indicated a good consistency between the predicted and observed probabilities of VA in both training and validation sets (Figure 4A and B).
 |
Table 4 Baseline Characteristics of the Patients in the Validation Group |
Discussion
In the present study, we analyzed the risk factors associated with malignant VA during hospitalization in patients with AMI. The VA prediction model based on these factors showed a high accuracy in the training and validation sets. It is convenient for clinicians to early and quickly stratify AMI patients.4,10,13,14
Malignant VA is one common cause of deaths (more than half are sudden) after AMI.15–18 The Grace Global Registry in May 2007 suggests that the incidence of malignant VA is 11.7% in STEMI patients and 4.9% in NSTEMI patients. For those patients with malignant VA in AMI patients, the mortality increases significantly, which is consistent with the results observed in our study. The incidence of malignant VA is still high in AMI patients, therefore resulting in a poor prognosis. So, our prediction model can help clinicians to take active preventive measures, individualize management and improve the prognosis of AMI patients.
In our prediction model, we found that Killip grade ≥3 at admission and LVEF < 50% were two strong predictors of VA risk, suggesting that cardiac function after myocardial injury is closely related to the risk of VA post AMI. Lee et al have also reported a similar conclusion in their cohort study. After AMI, the extensive death of cardiomyocytes leads to the formation of local scars in the myocardium, thus weakening cardiac systolic function and disrupting cardiac electrical activity. In addition, premature ventricular contraction is also an independent risk factor for malignant VA, suggesting that active measures should be taken to reduce the risk of VA and sudden death. For AMI patients with significantly worse cardiac functions or recurrent and persistent VA, early ICD implantation should be performed to reduce the risk of VA and death.19 Cardiac function may be improved significantly after revascularization therapy after AMI. In the present study, the patients who had undergone early ICD implantation were excluded. In a long follow-up, the value of ICD implantation for predicting the risk of VA should be fully considered, which may increase the accuracy of the present prediction model.
It is reported that electrolyte disorders are one main cause of cardiac electrical storms. We found that patients with hypokalemia are more likely to develop malignant VA, which suggests that electrolyte disorders, especially abnormal blood potassium and magnesium levels, should be intervened during the overall treatment of AMI.20–22 We also found that type-2 diabetes and baseline creatinine level were also strongly associated with the risk of VA in AMI patients.23–25 It has been reported that type 2 diabetes predicts a poor prognosis of AMI.26–28 Renal impairment in the early stage of AMI was also closely associated with an adverse prognosis in a short term.29–31 A recent study has reported that acute kidney injury in AMI patients is associated with a high mortality in a long term.32 Early identification of patients at a high risk of acute kidney injury is important, and an artificial intelligence model for predicting acute kidney injury risk has been developed.33 In another study, age, creatinine and ejection fraction scores showed a high predictive value for in-hospital mortality in cardiogenic shock patients.34 Cardiogenic shock increases the mortality in AMI patients. Early use of IABP can improve the prognosis of these patients.35 In our previous studies, we have also found that a lower systolic blood pressure level at admission and a low level of free triiodothyronine are independently associated with the short-term outcomes in patients with AMI.36–38 In the current study, hypotension and free triiodothyronine were not associated with the risk of VA. Therefore, more attention should be paid to blood glucose and renal function in patients with AMI.
There are also some limitations in the present study. First, the sample size is relatively small. The accuracy of the prediction model should be validated in a larger cohort. Second, this study is a retrospective study of patients from two centers. A prospective study based on more centers is needed to evaluate the accuracy of the model. Third, we only observed the occurrence of malignant VA in AMI patients in a short term. A long-term follow-up is still needed in the future study.
Conclusion
We have established a clinical prediction risk score for predicting the occurrence of VA in AMI patients. The prediction score is easy to use, performs well and can be used to guide clinical practice.
Funding
This study was supported by grants from Changzhou High-Level Medical Talents Training Project (2022CZBJ053, 2022CZBJ054), National Natural Science Foundation of China (Grant No.82270328), Natural Science Foundation of Jiangsu Province (BK20221229), Technology Development Fund of Nanjing Medical University (NMUB2020069), Major Research plan of Changzhou Health Commission of Jiangsu Province of China (ZD202215), China Postdoctoral Science Funding Program (2022M720544) and Changzhou Sci & Tech Program (CE20225051).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheang S, LeLorier P, Gajewski K. Diffuse ST-segment elevation with idiopathic malignant ventricular arrhythmia. Circulation. 2021;144(5):399–402. doi:10.1161/CIRCULATIONAHA.121.054910
2. Akhtar M, Lorenzini M, Pavlou M, et al. Association of left ventricular systolic dysfunction among carriers of truncating variants in filamin C with frequent ventricular arrhythmia and end-stage heart failure. JAMA Cardiol. 2021;6(8):891. doi:10.1001/jamacardio.2021.1106
3. Sun L, Mao L, Zou A, et al. 急性心肌梗死患者住院期间恶性心律失常风险临床预测模型的构建与验证 [Development and validation of a clinical predictive model for the risk of malignant ventricular arrhythmia during hospitalization in patients with acute myocardial infarction]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(4):438–442. Chinese. doi:10.3760/cma.j.cn121430-20201217-00760
4. Mizuguchi Y, Konishi T, Nagai T, et al. Prognostic value of admission serum magnesium in acute myocardial infarction complicated by malignant ventricular arrhythmias. Am J Emerg Med. 2021;44:100–105. doi:10.1016/j.ajem.2021.02.005
5. Zampella E, Mannarino T, Gaudieri V, et al. Effect of changes in perfusion defect size during serial stress myocardial perfusion imaging on cardiovascular outcomes in patients treated with primary percutaneous coronary intervention after myocardial infarction. J Nucl Cardiol. 2021;13:19.
6. Wang J, Gao H, Xiao J, et al. The clinical features and prognosis of type 4C myocardial infarction in patients with non-ST-segment elevation myocardial infarction. Ann Transl Med. 2021;9(14):1153. doi:10.21037/atm-21-2587
7. Watanabe S, Usui M. Serum uric acid level is associated with reperfusion ventricular arrhythmias in acute myocardial infarction. Diabetes Metabolic Syndrome. 2021;15(4):102198. doi:10.1016/j.dsx.2021.102198
8. Husková Z, Kikerlová S, Sadowski J, et al. Increased endogenous activity of the renin-angiotensin system reduces infarct size in the rats with early angiotensin II-dependent hypertension which survive the acute ischemia/reperfusion injury. Front Pharmacol. 2021;12:679060. doi:10.3389/fphar.2021.679060
9. Wang L, Li N, Wang F, et al. P2Y12 inhibition in macrophages reduces ventricular arrhythmias in rats after myocardial ischemia-reperfusion. Adv Clin Exp Med. 2021;30(4):413–420. doi:10.17219/acem/133139
10. Saito K, Kondo Y, Takahashi M, et al. Factors that predict ventricular arrhythmias in the late phase after acute myocardial infarction. ESC Heart Failure. 2021;8(5):4152–4160. doi:10.1002/ehf2.13499
11. Vallabhajosyula S, Kanwar S, Aung H, et al. Temporal trends and outcomes of left ventricular aneurysm after acute myocardial infarction. Am J Cardiol. 2020;133:32–38. doi:10.1016/j.amjcard.2020.07.043
12. Vallabhajosyula S, Patlolla SH, Verghese D, et al. Burden of arrhythmias in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2020;125(12):1774–1781. doi:10.1016/j.amjcard.2020.03.015
13. Xia W, Yang N, Li Y. Analysis of risk factors for adverse cardiovascular events in elderly patients with acute myocardial infarction and Non-Alcoholic Fatty Liver Disease (NAFLD). Med Sci Monit. 2020;26:e922913. doi:10.12659/MSM.922913
14. Tran H, Ash AS, Gore JM, et al. Twenty-five year trends (1986–2011) in hospital incidence and case-fatality rates of ventricular tachycardia and ventricular fibrillation complicating acute myocardial infarction. Am Heart J. 2019;208:1–10. doi:10.1016/j.ahj.2018.10.007
15. Su J, Fu X, Tian Y, et al. Additional predictive value of serum potassium to Thrombolysis In Myocardial Infarction risk score for early malignant ventricular arrhythmias in patients with acute myocardial infarction. Am J Emerg Med. 2012;30(7):1089–1094. doi:10.1016/j.ajem.2011.07.009
16. Liew R, Chiam P. Risk stratification for sudden cardiac death after acute myocardial infarction. Ann Acad Med Singapore. 2010;39(3):237–246. doi:10.47102/annals-acadmedsg.V39N3p237
17. Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36(4):1202–1209. doi:10.1016/S0735-1097(00)00865-2
18. Ito H, Taniyama Y, Iwakura K, et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol. 1999;33(3):654–660. doi:10.1016/S0735-1097(98)00604-4
19. Hayıroğlu M, Çınar T, Çinier G, et al. Comparison of mortality prediction scores in elderly patients with ICD for heart failure with reduced ejection fraction. Aging Clin Exp Res. 2022;34(3):653–660. doi:10.1007/s40520-021-01960-6
20. Shang W, Ma Q. Malignant arrhythmias as the unmasked manifestation of thyroid storm. Int J Gen Med. 2020;13:693–698. doi:10.2147/IJGM.S265833
21. Bilha S, Mitu O, Teodoriu L, et al. Thyrotoxic periodic paralysis-A misleading challenge in the emergency department. Diagnostics. 2020;10(5):316. doi:10.3390/diagnostics10050316
22. Adler C, Schregel F, Heller T, et al. Malignant arrhythmias during induction of target temperature management after cardiac arrest. Ther Hypothermia Temp Manag. 2020;10(4):229–236. doi:10.1089/ther.2019.0025
23. Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148. doi:10.1186/s12933-020-01127-z
24. Lee S, Jeevaratnam K, Liu T, et al. Risk stratification of cardiac arrhythmias and sudden cardiac death in type 2 diabetes mellitus patients receiving insulin therapy: a population-based cohort study. Clin Cardiol. 2021;44(11):1602–1612. doi:10.1002/clc.23728
25. Hu W, Zhang D, Tu H, et al. Reduced cell excitability of cardiac postganglionic parasympathetic neurons correlates with myocardial infarction-induced fatal ventricular arrhythmias in type 2 diabetes mellitus. Front Neurosci. 2021;15:721364. doi:10.3389/fnins.2021.721364
26. Oost L, van der Heijden AAWA, Vermeulen EA, et al. Serum magnesium is inversely associated with heart failure, atrial fibrillation, and microvascular complications in type 2 diabetes. Diabetes Care. 2021;44(8):1757–1765. doi:10.2337/dc21-0236
27. Kim Y, Her A-Y, Jeong MH, et al. Comparative effect of statin intensity between prediabetes and type 2 diabetes mellitus after implanting newer-generation drug-eluting stents in Korean acute myocardial infarction patients: a retrospective observational study. BMC Cardiovasc Disord. 2021;21(1):386. doi:10.1186/s12872-021-02198-w
28. Backhaus S, Kowallick JT, Stiermaier T, et al. Cardiac magnetic resonance myocardial feature tracking for optimized risk assessment after acute myocardial infarction in patients with type 2 diabetes. Diabetes. 2020;69(7):1540–1548. doi:10.2337/db20-0001
29. Chiang C, Huang S-C, Chen M, et al. Effects of renal impairment on cardiac remodeling and clinical outcomes after myocardial infarction. Int J Med Sci. 2021;18(13):2842–2848. doi:10.7150/ijms.61891
30. Gao S, Liu Q, Chen H, et al. Predictive value of stress hyperglycemia ratio for the occurrence of acute kidney injury in acute myocardial infarction patients with diabetes. BMC Cardiovasc Disord. 2021;21(1):157. doi:10.1186/s12872-021-01962-2
31. Clerico A, Padoan A, Zaninotto M, et al. Clinical relevance of biological variation of cardiac troponins. Clin Chem Lab Med. 2020;59:641–652.
32. Hayıroğlu M, Bozbeyoglu E, Yıldırımtürk Ö, et al. Effect of acute kidney injury on long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention in a high-volume tertiary center. Turk Kardiyol Dern Ars. 2020;48(1):1–9. doi:10.5543/tkda.2019.84401
33. Cai D, Xiao T, Zou A, et al. Predicting acute kidney injury risk in acute myocardial infarction patients: an artificial intelligence model using medical information mart for intensive care databases. Front Cardiovasc Med. 2022;9:964894. doi:10.3389/fcvm.2022.964894
34. Çinar T, Hayiroğlu Mİ, Şeker M, et al. The predictive value of age, creatinine, ejection fraction score for in-hospital mortality in patients with cardiogenic shock. Coron Artery Dis. 2019;30(8):569–574. doi:10.1097/MCA.0000000000000776
35. Hayıroğlu M, Çanga Y, Yıldırımtürk Ö, et al. Clinical characteristics and outcomes of acute coronary syndrome patients with intra-aortic balloon pump inserted in intensive cardiac care unit of a tertiary clinic. Turk Kardiyol Dern Ars. 2018;46(1):10–17. doi:10.5543/tkda.2017.11126
36. Wang Q, Qian W, Sun Z, et al. Nomograms based on pre-operative parametric for prediction of short-term mortality in acute myocardial infarction patients treated invasively. Aging. 2020;13(2):2184–2197. doi:10.18632/aging.202230
37. Zhou X, Sun Z, Zhuang Y, et al. Development and validation of nomogram to predict acute kidney injury in patients with acute myocardial infarction treated invasively. Sci Rep. 2018;8(1):9769. doi:10.1038/s41598-018-28088-4
38. Sun L, Zhu W, Ji Y, et al. Association of plasma free triiodothyronine levels with contrast-induced acute kidney injury and short-term survival in patients with acute myocardial infarction. Endocr Connect. 2022;11(7). doi:10.1530/EC-22-0120
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.