Back to Journals » Cancer Management and Research » Volume 12
A New Nomogram Based on Early Postoperative NLR for Predicting Infectious Complications After Gastrectomy
Authors Wang C , Huang H, He Y, Yu Y, Zhou Q, Wang R , He J, Han S
Received 14 November 2019
Accepted for publication 10 January 2020
Published 7 February 2020 Volume 2020:12 Pages 881—889
DOI https://doi.org/10.2147/CMAR.S238530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Chen Wang, Han-zhang Huang, Yu He, Yu-jun Yu, Qing-miao Zhou, Rong-jian Wang, Jian-bo He, Shao-liang Han
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China
Correspondence: Shao-liang Han
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, People’s Republic of China
Tel +86-577-88069307
Fax +86-577-88069555
Email [email protected]
Purpose: Our study aimed to construct a visible model to evaluate the risk of infectious complications after gastrectomy.
Methods: The clinical data of 856 patients who underwent gastrectomy were used to retrieve medical records. Univariate and multivariate analyses were performed to correlate early postoperative NLR and operative variables with postoperative complications, and the construction of the nomogram was based on logistic regression. The concordance index and receiver operating characteristic curves were used to evaluate the model performance.
Results: The postoperative infectious and noninfectious complication rates after gastrectomy were 18.5% (158/856 cases) and 12.3% (105/856 cases) respectively. Postoperative NLR (within 24 h) independently predicted the development of postoperative infectious complication. Multivariate analysis revealed that age, diabetes, body mass index (BMI), intraoperative blood transfusion and postoperative NLR were independent risk factors. The nomogram model showed a good performance in terms of predicting infectious complications after gastrectomy (concordance index=0.718).
Conclusion: Age, diabetes, BMI, intraoperative blood transfusion and postoperative NLR were independent risk factors of postoperative infectious complications after gastrectomy, and a novel nomogram based on these results can be used to predict postoperative infection and has the advantages of simple application and easy access.
Keywords: gastrectomy, infectious complication, nomogram, prediction, risk model
Introduction
Curative gastrectomy remains the most important treatment of gastric malignancies, including total gastrectomy, subtotal gastrectomy, and partial gastrectomy. It is reported that the morbidity of complications after gastrectomy ranges from 17.4% to 37.0%, with the rate of infectious complications between 17.6% and 19.8%.1–6 Postoperative infectious complications are associated not only with increased length of hospital stay and greater economic cost but also with tumor recurrence and poor prognosis in gastric cancers.2–5 Therefore, it is important to identify postoperative infectious complications in early stage and take corresponding treatment.
The neutrophil to lymphocyte rate (NLR) is an indicator of systemic inflammatory response, independently predicts poor prognosis after curative resection of several malignant neoplasms, including gastric cancer.7–10 However, numerous studies have focused on the relationship between the NLR or PLR and tumor characteristics or survival of malignancy; only few evidence suggest that preoperative NLR could independently predict postoperative infectious complications in both colorectal cancer and gastric cancer.2,11 Recently, a link between systemic inflammation and postoperative morbidity was identified by Moyes.11 However, the relationship between the perioperative systemic inflammatory response and postoperative complications in patients with curative gastrectomy has not been examined and remains uncertain. Therefore, our study aimed to analyze their association and to construct a visible model to evaluate the risk of infectious complications.
Materials and Methods
Patients’ Selection
From September 2016 to September 2018, 894 consecutive patients with gastric cancer who underwent gastrectomy at the Department of Gastrointestinal Surgery, the First Affiliated Hospital of Wenzhou Medical University were analyzed retrospectively. According to the exclusion criteria below, 856 patients were eventually enrolled in this study. Curative gastrectomy and lymph node dissection (D1 or D2) were performed according to the guidelines of the Japanese Gastric Cancer Association.12 All patients had prophylactic use of second-generation cephalosporin for 5 days on average after surgery. Data regarding clinical characteristics, surgery, pathology, and follow-up were retrospectively collected from our database.
Exclusion Criteria
The following patients had been ruled out from this study: (1) Patients who underwent preoperative chemotherapy (6 patients), emergency surgery (16 patients) or R1-R2 resection (7 patients) were excluded; (2) Patients who had evidence of infection or other inflammatory conditions before the surgery were excluded (9 patients).
Blood Analysis
Preoperative blood samples were collected at the first time after admission, while postoperative blood samples were collected within 24 h after surgery, which included hemoglobin, neutrophil, platelet and lymphocyte count, albumin, and tumor markers. The NLR was calculated from the blood sample by dividing the absolute neutrophil count by the absolute lymphocyte count. Similarly, PLR and OPNI were calculated indirectly from the above data.
Determination of NLR Cutoff Value
We determined the optimal discriminator value for NLR by using receiver operating characteristic (ROC) curve analysis. At each value, the sensitivity and specificity for each outcome under study were plotted, generating a ROC curve. The ratio closest to the point with maximum sensitivity and specificity was selected as the cutoff value.
Histological Diagnosis
Resected specimens were examined histopathologically and staged according to the International Union Against Cancer tumor-node-metastasis (TNM) classification.13 No patient had clinical evidence of infection or other inflammatory conditions at the time of sampling.
Classification of Postoperative Complications
According to the Clavien-Dindo classification, postoperative complications of grade 2 or higher occurred within 30 days after surgery was recorded.14 The information of complications was obtained from medical records including medical history, laboratory test and imaging examination. The postoperative complications were divided into two groups: infectious complications and non-infectious complications. Infectious complications mainly included intra-abdominal infection, anastomotic leakage, wound infection, and respiratory infection. Non-infectious complications mainly included intra-luminal bleeding, deep vein thrombosis, surgical gastroparesis syndrome, and bowel obstruction. The methods to diagnose postoperative complications were shown in Table S1. If multiple infectious complications occurred in a single patient, only the primitive or the most severe one was counted in the procedure of statistical analysis.
Definition of Infectious Complications
The criteria used to define postoperative infectious complications were referred to the criteria described previously.11 That is, (1) Wound infection was defined as superficial or deep infection with the presence of pus that required treatment with anti-microbacterical agents or wound drainage; (2) Intra-abdominal abscess was defined as abdominal fluid collection associated with fever or increased white blood cell count that discharged spontaneously or required surgery or ultrasonographically guided drainage, associating with positive microorganism on blood or fluid culture; (3) Respiratory tract infection was defined by respiratory symptoms and signs combined fever above 38.5°C and a positive X-ray findings, and requirement of antibiotic treatment; (4) Septicemia was defined by clinical symptoms combined with a positive blood culture.
Statistical Analysis
The Kolmogorov–Smirnov test was used to determine whether the variables obey normal distribution. The Mann–Whitney U-test was used to compare the non-normal distributed variables between the infectious complication group and the non-infectious complication group. In order to determine the cutoff points of variables, we plotted the receiver operating characteristic curves and the values with the maximal Youden index. According to the cutoff points, patients were divided into two groups. Univariate analysis was performed with the use of χ2 test. Based on the result of univariate analysis, a multivariate logistic regression analysis was used to confirm the independent risk factors and calculate the odds ratio and 95% confidence interval of each factor. With the use of the risk factors obtained in the multivariate analysis, the nomogram was plotted to assess the postoperative infectious complication probability. The calibration curve of the prediction model was plotted to compare the predicted and actual probability of postoperative infectious complications. We also calculated the concordance index (C-index) to evaluate the model performance. Receiver operating characteristic (ROC) curve was used to compare the performance of the nomogram and individual indicators.
All the statistical analyses and graphics were performed with IBM SPSS 23.0 (SPSS Inc, Armonk, NY) and RStudio software (version 1.2.1335- 2009-2019; RStudio, Inc.). P<0.05 was regarded as statistically significant.
Results
Patients’ Characteristics
Among total of 856 patients, 640 patients were male and 216 patients were female. The patients’ median age was 65 years, ranging from 58 years to 72 years. The tumor location was antrum in 433 cases (50.6%), body in 267 cases (31.2%), cardia in 131 cases (15.3%) and total stomach in 25 cases (2.9%). The surgical approach was laparoscopy in 301 patients (35.2%), and laparotomy in 555 patients (64.8%) (Table 1).
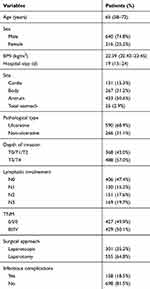 |
Table 1 Clinical and Pathological Characteristics |
Nutritional Status and Postoperative Early Blood Routine Index
There was no difference between patients with and without postoperative infectious complications in nutritional status before operation. Furthermore, it turned out there was also no difference in the postoperative early individual indicators such as WBC, lymphocyte count and neutrophil count between each group. However, the unit indicators including NLR and PLR were higher in patients with postoperative infectious complication (P<0.001) by comparison with those in patients without infectious complication (Table 2).
 |
Table 2 Blood Routine Index and Nutritional Status, According to Infectious Complication Involvement |
Postoperative Complications
After surgery, 262 patients (30.6%) developed postoperative complications, 158 cases (18.5%) of them were infectious complications, and 105 cases (12.3%) were non-infectious complications. Among infectious complications, the intra-abdominal infection without leakage was the most common infectious complication after gastrectomy (52 cases, 31.1%), followed by respiratory infection (36 cases, 21.6%), wound infection (26 cases, 15.6%), and intra-abdominal infection with leakage (25 cases, 15.0%) (Table 3).
 |
Table 3 Postoperative Complications After Gastrectomy |
Univariate and Multivariate Analysis
Univariate analyses were used to examine the variables of the development of postoperative infectious and noninfectious complications. As the results of chi-square: age (χ2=11.279, P=0.001), BMI (χ2=3.978, P=0.046), diabetes (χ2=10.057, P=0.002), preoperative OPNI (χ2=4.663, P=0.031), depth of invasion (χ2=4.504, P=0.034), lymphatic invasion (χ2=11.484, P=0.009), reconstruction method (χ2=10.110, P=0.018), postoperative NLR (χ2=26.736, P<0.001), postoperative PLR (χ2=16.578, P<0.001), laparoscopy (χ2=3.795, P=0.051), and intraoperative blood transfusion (χ2=17.424, P<0.001) were significantly different between patients with and without postoperative infectious complications (Table 4). This suggested that these variables were potential independent risk factors. Based on these variables, a multivariate logistic regression analysis was performed. On multivariate analysis, high postoperative NLR (OR=2.194, P<0.001), age (OR=1.529, P=0.037), diabetes (OR=2.429, P=0.002), body mass index (OR=1.599, P=0.042) and intraoperative blood transfusion (OR=2.362, P=0.006) were significantly associated with postoperative infectious complications (Table 5). Taken together, age, diabetes, body mass index (BMI), intraoperative blood transfusion and postoperative NLR were the independent predictive indicators of postoperative infectious complications.
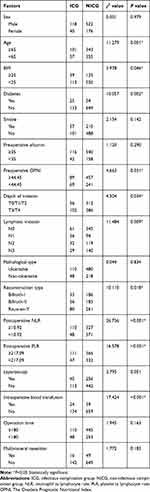 |
Table 4 Univariate Analysis of the Risk of Postoperative Infectious Complications After Gastrectomy |
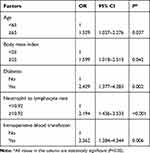 |
Table 5 Multivariate Analysis of the Risk of Postoperative Infectious Complications After Gastrectomy |
Construction of the Nomogram for Infectious Complication
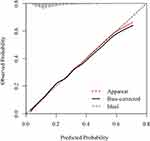
Based on the multivariate analysis above, the values of age, diabetes, BMI, intraoperative blood transfusion and postoperative NLR were assigned a corresponding score; subsequently, a novel nomogram was constructed to predict the infectious complication after gastrectomy (Figure 1). The scores of each subgroup variable were summarized to obtain a total score which matches a corresponding infectious complication risk. The C-index of this model was 0.718 and Calibration was used to verify the performance of the model (Figure 2). To compare the performance of the model with single indicators, we plotted the ROC curve for the nomogram, postoperative NLR, postoperative neutrophil, and postoperative lymphocyte. The area under the ROC curve of these indicators was 0.718 (nomogram), 0.641 (pNLR), 0.615 (pNeutrophil), and 0.562 (pLymphocyte), respectively (Figure 3). Our nomogram showed more reliable discrimination ability as a predictive indicator for infectious complication after gastrectomy.
Discussion
Gastrectomy is still the main treatment for gastric tumors, and it is reported that the morbidity after gastrectomy ranges from 17.4% to 37.0%, including the rate of infectious complications from 17.6% to 19.8%.1–6 In this study, postoperative complications developed in 262 patients (30.6%) after gastrectomy, including infectious complications of 18.5% (158 patients) and non-infectious complications of 12.3% (105 patients), which is similar to the previous studies.1–5 The main postoperative infectious complications after gastric operation include intra-abdominal infection, wound infection, anastomosis leakage, and respiratory infection.1–3 The infectious complications not only increase the length of postoperative hospital stay and financial burden of patients, but also even affect the long-term prognosis of the patient.2–5 Usually, with the use of antibiotics and local drainage, the treatment of infectious complications can achieve a good therapeutic effect. Therefore, how to identify infectious complications in early stage is of great significance.
Neutrophil to lymphocyte rate (NLR) is an indicator of systemic inflammatory response, and it is very simple to calculate in clinical practice without extra costs. Previous studies have shown that preoperative NLR is correlated with postoperative long-term prognosis of various tumors including gastric cancer, colorectal cancer, hepatic cancer, and breast cancer.7–10 Evidence suggest that preoperative NLR could independently predict postoperative infectious complications in colorectal cancer and gastric cancer.2,11 Mohri2 reported that preoperative NLR was independently associated with the development of postoperative infectious complication after gastrectomy, but not associated with the development of noninfectious complication. In this study, we found that early postoperative NLR (within 24 h) was associated with postoperative infectious complications, and NLR (AUC=0.641) was better than neutrophil (AUC=0.615) in predicting postoperative infectious complications.
The Onodera prognostic nutritional index (OPNI) which was initially presented by Onodera, calculated as 10×serum albumin (g/dl) +0.005×total lymphocyte count, is an indicator of nutritional status.15 OPNI has been used to assess the outcomes in several cancers in previous studies with promising results, it reported that a low OPNI leads to poorer outcomes.16–18 However, the correlation between OPNI and short-term outcomes after gastrectomy is not clear. Our study showed that preoperative OPNI was not correlated with the incidence of postoperative infectious complications.
Hamilton19 reported that major morbidity increased with age, from 16.3% (<65 years old) to 21.5% (76–80 years old), and 24.1% (>80 years old) (P<0.001) after gastrectomy in 3637 patients, driven by higher respiratory and infectious events. Other studies showed the increasing postoperative morbidity rate with age.6,20,21 In this study, older age was independently associated with increased risk of infectious complication after gastrectomy (OR=1.529, P=0.037). People with diabetes have an increased susceptibility to infection, and diabetes confers an increased risk of developing and dying from an infectious disease.22 The high blood glucose fluctuation and poor postoperative blood glucose control in diabetic patients were associated with infectious complications after surgery23,24; Olsen25 reported that diabetes was associated with the highest independent risk of spinal surgical site infection. In this study, the incidence of postoperative infectious complications was higher in patients with diabetes than that in patients without diabetes (OR=2.429, P=0.002).
It has been reported that blood transfusion is the risk factor associated with the incidence of postoperative infectious complications after gastrectomy.26–28 In this study, we found that patients who received intraoperative blood transfusion were more likely to occur postoperative infectious complications (OR =2.362 P =0.006). Xiao29 analyzed 1835 patients who underwent gastrectomy for gastric cancer, they found BMI≥25 kg/m2 was the risk factor of postoperative intra-abdominal infection (OR=1.968, 95% CI: 1.107–3.500, P= 0.021). Previous studies also demonstrated that BMI ≥25 kg/m2 was related to the incidence of surgical site infection (SSI).28,30 Our results indicated that BMI≥25 kg/m2 was an independent risk factor of postoperative infectious complications after gastrectomy (OR=1.599, P=0.042).
Studies have shown that there was no significant difference in the overall morbidity of infectious complications after laparoscopic or open gastrectomy.31,32 In this study, 301 patients (35.2%) underwent laparoscopic surgery, and 555 patients (64.8%) underwent laparotomy. Although the overall morbidity of infectious complication has no difference, laparoscopic surgery means lower local infectious complications (especially wound infection) and shorter hospital stay. On univariate analysis, the postoperative infectious complication rate in laparoscopic surgery patients was lower than that in laparotomy patients (P=0.051). However, laparoscopy was not an independent risk factor for postoperative infectious complications in multivariate analysis.
Nomogram is a good visual model that combines multiple indicators to predict disease prognosis and has been used in a variety of cancers.33–36 In this study, age, diabetes, BMI, intraoperative blood transfusion and postoperative NLR were independent risk factors for predicting the postoperative infectious complications after gastrectomy on multivariate analysis. Based on these results, we constructed a nomogram, and it showed more reliable discrimination ability as a predictive indicator for infectious complication after gastrectomy, the C-index of this model was 0.718. To the best of our knowledge, this is the first study to verify the relationship between early postoperative NLR and the infectious complications after gastrectomy. Although our model does not target a specific infectious complication, it has universal significance to predict all infectious complications. In addition, these complications (such as pulmonary infection, incisional infection and intra-abdominal infection) ultimately are needed to be intervened with strong antibiotics. The advantage of our model is that the data is easy to obtain and the risk of infection can be assessed within 24 hrs after the operation. Therefore, the model may be used to alert clinicians to take appropriate measures in reducing the incidence of postoperative infectious complications. When the risk of infection is assessed to be greater than 50%, we recommend prophylactic use of strong antibiotics after surgery (such as third-generation cephalosporin), and better control of blood glucose for diabetes patients. Nevertheless, there are still many deficiencies in this study. Firstly, all patients were from a single-institution, not a multicenter study; Secondly, this was a retrospective study which needs further verification in prospective research.
In conclusion, age, diabetes, BMI, intraoperative blood transfusion and early postoperative NLR were independent risk factors of postoperative infectious complications after gastrectomy, and a novel nomogram based on these results can be used to predict postoperative infection and has the advantages of simple application and easy access.
Ethics Statement
This study was reviewed and approved by the Institutional Review Board (IRB) of The First Affiliated Hospital of Wenzhou Medical University as a retrospective study. This study was conducted in accordance with the Declaration of Helsinki. The requirement for patient-informed consent was waived because of the retrospective nature of this study, and the patients’ data confidentiality was protected.
Funding
This work was financially supported by the grant from Zhejiang Provincial Natural Science Foundation (Grant No. Y15H160192 http://www.zjnsf.gov.cn).
Acknowledgment
We thank professor Song-Rong Ji from Department of Foreign Language, Wenzhou University, for checking the language of this paper.
Author Contributions
All authors contributed to conception and design, acquisition, or analysis of data; participate in drafting and revising the article; gave final approval of the version to be published and agree to be accountable for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Watanabe M, Miyata H, Gotoh M, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg. 2014;260(6):1034–1039. doi:10.1097/SLA.0000000000000781
2. Mohri Y, Tanaka K, Toiyama Y, et al. Impact of preoperative neutrophil to lymphocyte ratio and postoperative infectious complications on survival after curative gastrectomy for gastric cancer: a single institutional cohort study. Medicine (Baltimore). 2016;95(11):e3125. doi:10.1097/MD.0000000000003125
3. Wang S, Xu L, Wang Q, et al. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: a systematic review and meta-analysis of observational studies. World J Surg Oncol. 2019;17(1):52. doi:10.1186/s12957-019-1593-9
4. Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14(9):2559–2566. doi:10.1245/s10434-007-9434-4
5. Fujiya K, Tokunaga M, Mori K, et al. Long-term survival in patients with postoperative intra-abdominal infectious complications after curative gastrectomy for gastric cancer: a propensity score matching analysis. Ann Surg Oncol. 2016;23(Suppl 5):809–816. doi:10.1245/s10434-016-5577-5
6. Nelen SD, Bosscha K, Lemmens V, Hartgrink HH, Verhoeven RHA, de Wilt JHW; Dutch Upper Gastrointestinal Cancer Audit g. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg. 2018;105(9):1163–1170. doi:10.1002/bjs.10836
7. Szor DJ, Roncon Dias A, Pereira MA, et al. Neutrophil-lymphocyte ratio is associated with prognosis in patients who underwent potentially curative resection for gastric cancer. J Surg Oncol. 2018;117(5):851–857. doi:10.1002/jso.v117.5
8. Zhang J, Zhang HY, Li J, Shao XY, Zhang CX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(40):68837–68846. doi:10.18632/oncotarget.18575
9. Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. 2017;44(3):967–981. doi:10.1159/000485396
10. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2.
11. Moyes LH, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100(8):1236–1239. doi:10.1038/sj.bjc.6604997
12. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. doi:10.1007/s10120-016-0622-4
13. Brierley JD, Wittekind C. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors.
14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
15. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005.
16. Broggi MS, Patil D, Baum Y, et al. Onodera’s prognostic nutritional index as an independent prognostic factor in clear cell renal cell carcinoma. Urology. 2016;96:99–105. doi:10.1016/j.urology.2016.05.064
17. Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer. 2014;66(8):1362–1370. doi:10.1080/01635581.2014.956261
18. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389–3397. doi:10.1007/s13277-014-2973-y
19. Hamilton TD, Mahar AL, Haas B, et al. The impact of advanced age on short-term outcomes following gastric cancer resection: an ACS-NSQIP analysis. Gastric Cancer. 2018;21(4):710–719. doi:10.1007/s10120-017-0786-6
20. Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92(9):1099–1102. doi:10.1002/bjs.4952
21. Shin HS, Oh SJ, Suh BJ. Factors related to morbidity in elderly gastric cancer patients undergoing gastrectomies. J Gastric Cancer. 2014;14(3):173–179. doi:10.5230/jgc.2014.14.3.173
22. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi:10.2337/diacare.26.2.510
23. Vogel TR, Smith JB, Kruse RL. The association of postoperative glycemic control and lower extremity procedure outcomes. J Vasc Surg. 2017;66(4):1123–1132. doi:10.1016/j.jvs.2017.01.053
24. Kiran RP, Turina M, Hammel J, Fazio V. The clinical significance of an elevated postoperative glucose value in nondiabetic patients after colorectal surgery: evidence for the need for tight glucose control? Ann Surg. 2013;258(4):
25. Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90(1):62–69. doi:10.2106/JBJS.F.01515
26. Migita K, Takayama T, Matsumoto S, et al. Risk factors for surgical site infections after elective gastrectomy. J Gastrointest Surg. 2012;16(6):1107–1115. doi:10.1007/s11605-012-1838-1
27. Pinto V, Baldonedo R, Nicolas C, Barez A, Perez A, Aza J. Relationship of transfusion and infectious complications after gastric carcinoma operations. Transfusion. 1991;31(2):114–118. doi:10.1046/j.1537-2995.1991.31291142940.x
28. Tu RH, Huang CM, Lin JX, et al. A scoring system to predict the risk of organ/space surgical site infections after laparoscopic gastrectomy for gastric cancer based on a large-scale retrospective study. Surg Endosc. 2016;30(7):3026–3034. doi:10.1007/s00464-015-4594-y
29. Xiao H, Xiao Y, Quan H, Liu W, Pan S, Ouyang Y. Intra-abdominal infection after radical gastrectomy for gastric cancer: incidence, pathogens, risk factors and outcomes. Int J Surg. 2017;48:195–200. doi:10.1016/j.ijsu.2017.07.081
30. Hirao M, Tsujinaka T, Imamura H, et al.; Osaka Gastrointestinal Cancer Chemotherapy Study G. Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013;16(2):239–244. doi:10.1007/s10120-012-0174-1
31. Shi Y, Xu X, Zhao Y, et al. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2018;32(5):2427–2433. doi:10.1007/s00464-017-5942-x
32. Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350–1357. doi:10.1200/JCO.2015.63.7215
33. Aziz A, May M, Burger M, et al.; group Pr. Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort. Eur Urol. 2014;66(1):156–163. doi:10.1016/j.eururo.2013.12.018
34. Liu Z, Dai T, Wang Z, Zhang Z, Qiu W, He Y. Nomogram model to predict postoperative infection after mandibular osteoradionecrosis surgery. Sci Rep. 2017;7(1):3479. doi:10.1038/s41598-017-03672-2
35. Mao CC, Chen XD, Lin J, et al. A novel nomogram for predicting postsurgical intra-abdominal infection in gastric cancer patients: a prospective study. J Gastrointest Surg. 2018;22(3):421–429. doi:10.1007/s11605-017-3580-1
36. Chen L, Cai BB, Zhou CJ, et al. A sample model established by S-index predicting overall survival after curative resection of primary hepatocellular carcinoma. Cancer Manag Res. 2019;11:693–703. doi:10.2147/CMAR.S193593
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.