Back to Journals » Infection and Drug Resistance » Volume 15
A Neonate with Bacterial Meningitis Due to Vertically Transmitted Scrub Typhus
Authors Gao J, Liu T, Xiong X, Zhao M, Du K, Li J
Received 17 June 2022
Accepted for publication 10 September 2022
Published 18 September 2022 Volume 2022:15 Pages 5463—5467
DOI https://doi.org/10.2147/IDR.S378430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jin Gao,1,2 Tingting Liu,1 Xingyu Xiong,1 Mei Zhao,1 Kun Du,1,2 Jiwei Li2,3
1Department of Neonates; 2Yunnan Province Clinical Research Center for Children’s Health and Disease, Kunming, People’s Republic of China; 3Department of Pathology, Kunming Children’s Hospital, The Affiliated Children’s Hospital of Kunming Medical University, Kunming, People’s Republic of China
Correspondence: Kun Du; Jiwei Li, Email [email protected]; [email protected]
Abstract: Scrub typhus is a zoonotic disease caused by Orientia tsutsugamushi, which is transmitted by larval trombiculid mites. Due to nonspecific clinical presentation, scrub typhus is grossly underdiagnosed in pregnant women, fetuses and neonates. Here, we present a congenital infection case and hope to provide more insight into this disease.
Keywords: neonatal scrub typhus, vertical transmission, congenital infection, Orientia tsutsugamushi, meningitis
Plain Language Summary
A case of scrub infection during the third trimester of pregnancy with subsequent vertical transmission to the fetus.
Introduction
Scrub typhus is a zoonotic disease caused by the gram-negative obligate intracellular pathogen Orientia tsutsugamushi (Rickettsia species),1 which is transmitted by larval trombiculid mites (chiggers), and humans are incidental hosts.2 Approximately one million children are infected annually,3 but evidence of vertical transmission to fetuses has rarely been seen. To our knowledge, there are only 3 cases of congenital infection of scrub typhus that have been reported in the literature (Table 1).4–6 Here, we present another congenital infection case.
 |
Table 1 Demographic Characteristics, Symptoms, Laboratory Characteristics and Prognoses of Neonates and Mothers with Scrub Typhus |
Case
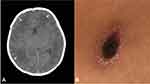
A 10-day-old male presented with high-grade fever and jaundice for six days and seizures for two days. On examination, the baby was febrile, irritable, icteric, and hepatomegaly without eschar, rash, or petechiae. The abdomen was distended. The axillary temperature was 39.3 °C, the pulse rate was 176/min, and the respiration rate was 46/min. A complete blood count showed that the white blood cells (WBCs) count was 24.76×109/L with 76.4% lymphocytes, 12.1% neutrophils, and 8.1% monocytes. Tests showed that the total bilirubin level was 87.4 µmol/L, aspartate aminotransferase level was 336.6 U/L, alanine aminotransferase level was 1317.1 U/L, and lactate dehydrogenase level was 1477.6 U/L. Cerebrospinal fluid (CSF) examination showed a WBC count at 283×106/L with 95.1% mononuclear cells, 4.9% polymorphonuclear neutrophils, a protein concentration of 3.084 g/L and a glucose concentration of 1.18 mmol/L. Computed tomography (CT) showed patchy low-density areas in the bilateral frontal, temporal, and parietal lobes (Figure 1A). Tests for malaria, dengue, toxoplasmosis, leptospirosis, mycobacterium tuberculosis, rubella, cytomegalovirus, herpes simplex virus, and Japanese encephalitis virus were negative. A diagnosis of neonatal bacterial meningitis was made, and the patient was started on cefotaxime sodium combined with ampicillin and phenobarbital to control fever and seizures. On the third day of admission, his seizures and jaundice had subsided, but the fever persisted, and the CSF still showed abnormalities. We re-evaluated the family history and found that the patient’s mother had anemia (Hb: 73 g/L) since the 28th week of pregnancy and reversed fever of unknown origin from three days before delivery to postpartum. On examination, there was an 1.2×0.8 cm eschar on her left groin (Figure 1B). The Weil–Felix test was positive for OXK 1:640 and 1:160 in the mother and baby, respectively, and both had positive IgM-ELISA 1:160 to the Karp antigen.7 Meanwhile, Metagenomic next-generation sequencing (mNGS) was positive for Orientia tsutsugamushi in both mother and baby8 (Supplementary Material). The final diagnosis of the baby was congenital infection of scrub typhus with bacterial meningitis. His treatment was immediately adjusted to intravenous azithromycin 10 mg/kg/day for three weeks and oral azithromycin for one week, and his mother was treated with oral tetracycline. After treatment, the baby and his mother’s condition improved and stabilized. During the 4 weeks of hospitalization, the EEG, MRI, psychomotor assessment and Griffiths assessment were normal. No neurological sequelae were found after 6 months of follow-up. Written informed consent was obtained from the child’s parents.
Discussion
China is one of the main epidemic areas of tsutsugamushi disease, especially in Yunnan and Guangdong Provinces.9,10 The annual incidence rate is 2.46/100,000 and the incidence rate in children aged 0–9 years is 4.8/100,000. The areas with the highest incidence are located in southwestern Yunnan Province, including Baoshan City, Dehong Prefecture, Lincang City, and Xishuangbanna Prefecture, with an incidence rate as high as 52.48/100,000.11 The diagnosis of scrub typhus requires a comprehensive consideration of exposure history, clinical manifestations, serological tests, and organism isolation. According to the 8th edition of Infectious Diseases in China,12 the diagnostic criteria of tsutsugamushi disease should meet the following conditions: (1) The patient has been to the epidemic area in the epidemic season, and has a history of field work or sitting in the grass; (2) The clinical manifestations include fever, eschar or ulcer, local lymphadenopathy, rash, hepatosplenomegaly; (3) WBC count was decreased or normal in laboratory examination, and the agglutination reaction of Bacillus proteus OXK strain was positive (detection of serum OXK antibody by Weil-Felix test), and the titer gradually increased with the course of disease. The laboratory-based diagnoses of tsutsugamushi disease, besides the Weil-Felix test, include indirect immunofuorescence assays, indirect immunoperoxidase assays, enzyme-linked immunosorbent assay (ELISA), immunochromatographic tests (ICT), polymerase chain reaction (PCR) and mNGS. Among all assays, molecular-based approaches like PCR and mNGS have more specificity and sensitivity.7 The characteristics of clinical symptoms in children with scrub typhus are nonspecific, especially during the acute phase, with nonreactive serological tests, which lead to misdiagnosis or even complications with a high mortality rate.13 Therefore, it is necessary to require a high index of suspicion for diagnosis of scrub typhus in children and to provide timely treatment. According to the summary of four congenital cases, we identified some characteristics and developed some questions. First, approximately 50–80% of patients only have nonspecific symptoms without eschar or rash. Delivery in the epidemic area may be the only clue to track exposure history. Therefore, it is necessary to investigate the life history of pregnant women in detail. Second, there is a relationship between scrub typhus infection in pregnant women and fetal outcomes: pregnant women have a higher risk of infection-induced fetal loss and premature delivery in the first and/or second trimesters.14 Therefore, the prevention and intervention of scrub typhus during pregnancy can reduce the risk of fetal and neonatal outcomes. Third, elevated serum IgM levels provided direct evidence of intrauterine infection through the placenta or infection through perinatal blood-born transmission. O. tsutsugamushi can invade the vascular endothelium, leading to plasma leakage and end-organ ischemia;6 however, the vasculitis-associated pathogenic mechanisms of the placenta are unknown and may be associated with thrombotic occlusions and/or coagulopathy.15 Fourth, all congenital patients have severe complications, which may also be indirect evidence of congenital scrub typhus originating from prolonged intrauterine infection and/or untimely diagnosis.
Conclusion
Due to nonspecific clinical presentation, limited awareness, a low index of suspicion among clinicians, and a lack of diagnostic facilities, scrub typhus is grossly underdiagnosed in pregnant women, fetuses, and neonates. Further research is therefore needed to provide more clues for this disease, especially in epidemic areas.
Ethics
A parent of the patient provided informed consent for the case details and images to be published.
Acknowledgments
The manuscript is an original work and has not been published or under consideration for publication in another journal. The study complies with current ethical considerations. The authors confirm that all the listed authors have participated actively in the research and have seen and approved the submitted manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Kunming Health and Family Planning Commission Project grant 2020-SW-31. Kunming Health and Family Planning Commission Project grant 2019-SW-33. Yunnan Province’s Reserve Medical Talents Project grant H-2019002. Kunming Medical University Applied Basic Research Joint Special Project grant 202001AY070001-170.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xu G, Walker DH, Jupiter D, et al. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11(11):e0006062. doi:10.1371/journal.pntd.0006062
2. Rapmund G. Rickettsial disease of the Far East: new perspectives. J Infect Dis. 1984;149(3):330–338. doi:10.1093/infdis/149.3.330
3. Suprit B, Arpan S, Sumantra S, et al. Clinical profile and therapeutic response of scrub typhus in children: a recent trend from Eastern India. J Trop Pediatr. 2019;65(2):139–146. doi:10.1093/tropej/fmy027
4. Wang CL, Yang KD, Cheng SN, Chu ML. Neonatal scrub typhus: a case report. Pediatrics. 1992;89(5):965–968. doi:10.1542/peds.89.5.965
5. Suntharasaj T, Janjindamai W, Krisanapan S. Pregnancy with scrub typhus and vertical transmission: a case report. J Obstet Gynaecol Res. 1997;23(1):75–78. doi:10.1111/j.1447-0756.1997.tb00809.x
6. Shailja V, Gupta RK, Gupta ML. Scrub typhus causing neonatal hepatitis with acute liver failure-A case series.[J]. Indian J Gastroenterol. 2017;36(3):239–242. doi:10.1007/s12664-017-0761-5
7. Deepak K, Shagun G, Rupak N, et al. Diagnosis of scrub typhus: recent advancements and challenges. Biotech. 2020;10(9):396.
8. Qing M, Yuyan M, Qingqing W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(2):S231–S240. doi:10.1093/cid/ciy693
9. Yuan QH, Chen M, Yang XD. Epidemiological analysis of tsutsugamushi disease in Yunnan Province from 2006 to 2017. Chin J Vector Biol Control. 2018;29(6):4.
10. Peng J, Liao Y, Zhang M, Den AP, Zhang YT, Peng ZQ. Analysis on epidemic characteristics of tsutsugamushi disease in Guangdong Province from 2006 to 2017. S China J Prev Med. 2020;46(5):4.
11. Cao J, Hong-Xiang YA, Yuan QH, et al. Hierarchical cluster analysis on the incidences of scrub typhus in Yunnan Province, China, 2006–2014. Chin J Zoonoses. 2015;31(8):714–723.
12. Li LJ, Ren H. Infectious Diseases. Beijing: Peoples’s Medical Publishing House; 2013:140–144.
13. Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23(1):39–43. doi:10.1016/j.ijid.2014.02.009
14. Rajan SJ, Sathyendra S, Mathuram AJ. Scrub typhus in pregnancy: maternal and fetal outcomes.[J]. Obstet Med. 2016;9(4):164–166. doi:10.1177/1753495X16638952
15. Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6(1):e1466. doi:10.1371/journal.pntd.0001466
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.