Back to Journals » Clinical Epidemiology » Volume 9
A nationwide population-based cross-sectional survey of health-related quality of life in patients with myeloproliferative neoplasms in Denmark (MPNhealthSurvey): survey design and characteristics of respondents and nonrespondents
Authors Brochmann N, Flachs EM, Christensen AI , Andersen CL, Juel K , Hasselbalch HC, Zwisler AD
Received 19 July 2016
Accepted for publication 18 October 2016
Published 2 March 2017 Volume 2017:9 Pages 141—150
DOI https://doi.org/10.2147/CLEP.S117587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Nana Brochmann,1 Esben Meulengracht Flachs,2 Anne Illemann Christensen,3 Christen Lykkegaard Andersen,1 Knud Juel,3 Hans Carl Hasselbalch,1 Ann-Dorthe Zwisler4
1Department of Hematology, Zealand University Hospital, University of Copenhagen, Roskilde, 2Department of Occupational and Environmental Medicine, Bispebjerg University Hospital, Copenhagen, 3National Institute of Public Health, University of Southern Denmark, Copenhagen, 4Danish Knowledge Centre for Rehabilitation and Palliative Care, University of Southern Denmark and Odense University Hospital, Odense, Denmark
Objective: The Department of Hematology, Zealand University Hospital, Denmark, and the National Institute of Public Health, University of Southern Denmark, created the first nationwide, population-based, and the most comprehensive cross-sectional health-related quality of life (HRQoL) survey of patients with myeloproliferative neoplasms (MPNs). In Denmark, all MPN patients are treated in public hospitals and treatments received are free of charge for these patients. Therefore, MPN patients receive the best available treatment to the extent of its suitability for them and if they wish to receive the treatment. The aims of this article are to describe the survey design and the characteristics of respondents and nonrespondents.
Material and methods: Individuals with MPN diagnoses registered in the Danish National Patient Register (NPR) were invited to participate. The registers of the Danish Civil Registration System and Statistics Denmark provided information regarding demographics. The survey contained 120 questions: validated patient-reported outcome (PRO) questionnaires and additional questions addressing lifestyle.
Results: A total of 4,704 individuals were registered with MPN diagnoses in the NPR of whom 4,236 were eligible for participation and 2,613 (62%) responded. Overall, the respondents covered the broad spectrum of MPN patients, but patients 70–79 years old, living with someone, of a Danish/Western ethnicity, and with a higher level of education exhibited the highest response rate.
Conclusion: A nationwide, population-based, and comprehensive HRQoL survey of MPN patients in Denmark was undertaken (MPNhealthSurvey). We believe that the respondents broadly represent the MPN population in Denmark. However, the differences between respondents and nonrespondents have to be taken into consideration when examining PROs from the respondents. The results of the investigation of the respondents’ HRQoL in this survey will follow in future articles.
Keywords: survey design, cross-sectional survey, nationwide survey, patient-reported outcomes, health-related quality of life, myeloproliferative neoplasms
Introduction
The chronic myeloproliferative neoplasms (MPNs) encompass two disease groups: 1) chronic myeloid leukemia (CML) with the Philadelphia chromosome and 2) the main diseases essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF) without the Philadelphia chromosome.1 The diseases are caused by acquired damage to hematopoetic stem cells.1 The nature of MPNs is a chronic disease course, but mean lifespan is decreased, which is most pronounced in MF patients.2,3 Philadelphia-negative MPNs may change from an early cancer stage (ET and PV) to an advanced cancer stage (MF) with intervening transitional stages.4,5 CML may turn into blast crisis.6 Philadelphia-negative MPNs may also turn into blast crisis/acute myeloid leukemia (AML).1 For MPN patients who develop blast crisis/AML, the mean survival is only a few months.7,8 Previously, the management of these diseases aimed to relieve symptoms and to reduce the risk of thrombotic complications.8 Allogeneic stem cell transplantation is potentially curative, but unfortunately this treatment possibility only exists for a minority of patients.8,9 However, due to increased knowledge of specific genetic defects and molecular properties, a new targeted medical treatment era has evolved. Because of targeted treatment, overall survival of CML patients is prolonged significantly and, hopefully, the lifespan approaches that of the general population.10,11 Targeted treatment for ET, PV, and MF patients is expected to prolong overall survival.12–16
Previous studies have investigated health-related quality of life (HRQoL) in MPN patients.17–41 The presentation of symptoms depends on the MPN subtype, and the symptom profiles vary greatly from asymptomatic to burdensome.17–41 Given the knowledge of burdensome symptom profiles and impaired HRQoL in many MPN patients, a comprehensive patient-reported outcome (PRO) survey of a large, representative MPN population is warranted. A more extensive understanding of the patients’ health conditions and the influences on quality of life is likely to improve both medical treatments and thus prevent disease progression and worsening of symptoms, rehabilitation, and care. Given that CML patients have an overall longer lifespan that possibly approaches the lifespan of the general population in the new targeted treatment era,3 it is relevant to investigate whether the HRQoL of these patients correspondingly approach those of the general population.
In Denmark, all MPN patients are treated in public hospitals. The diagnoses of all patients are obtained in the hospital, and diagnosis codes are recorded in the National Patient Register (NPR).42,43 As the treatment is free of charge for MPN patients, majority of these patients receive the best available treatment. The best available treatment may not be suitable for a minority of MPN patients and there may be some MPN patients who do not wish to receive the best available treatment. We decided to undertake the first nationwide, population-based, and the most comprehensive HRQoL survey of MPN patients, who receive the best available treatment to the extent of its suitability for the patients and if they wish to receive the treatment. The PROs will be adjusted for potential confounding variables based on information from national registers.42–45
Objective
The purpose of this article is to present the survey design and the results of an examination of whether the respondents are representative of the entire MPN population in Denmark prior to analyzing PROs from the respondents. The results of the PRO examination will follow in future articles. Furthermore, this article with description of the survey design and characteristics of the respondents and nonrespondents to the survey will serve as a reference for future research articles because the MPN population and the PROs from the MPNhealthSurvey will serve as mainstays for future research.
Material and methods
Participants
The inclusion criteria were the following: 1) An MPN diagnosis of ET, PV, MF, unclassified MPN, or CML according to international diagnostic criteria9,11 registered in the NPR43 between 1977 (the year of creation) and March 31, 2013. The diagnosis codes in the NPR are based on the International Classification of Diseases (ICD). ICD-8 was used from 1977 to 1993, and ICD-10 was used from 1994 to 2013 (Table 1). Some ICD-10 MPN diagnosis codes changed between 1994 and 2013. A patient’s MPN diagnosis may change due to disease progression,4,5 and a change in diagnosis is reported to the NPR. Therefore, if more than one diagnosis of MPN was registered for a patient, the last registered diagnosis was used. Denmark has a Cancer Register. All patients with diagnoses of cancer must be registered in the NPR, and all of these registrations are automatically passed on to the Cancer Register. We decided to use the NPR to identify the MPN patients for this survey. 2) The MPN diagnosis had to have been registered at least once at a department of hematology or at a department of internal medicine that treats or previously treated MPN patients in Denmark. Today, MPN patients are almost exclusively treated in departments of hematology. 3) The patient had to be 16 years or older on September 4, 2013. 4) The patient had to be alive on September 4, 2013.
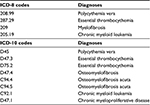  | Table 1 The survey population was formed from the following diagnoses Abbreviation: ICD, international classification of diseases. |
Data collection
The names and personal identification numbers of all individuals in Denmark with MPN diagnoses were extracted from the NPR on September 4, 2013. All postal addresses were obtained from the Civil Registration System (CRS).42,44,45 The patients who were alive, fulfilled all of the inclusion criteria, and who were not protected from inquiries during scientific surveys received a postal letter that was mailed on September 11, 2013. The mailing contained an introduction letter, a survey booklet, a prepaid stamped envelope, and a unique username for online login. The letter invited the patients to participate in the survey, described the survey’s purpose and content, and emphasized that participation was voluntary. A mixed-mode approach was used to collect the data. The respondents either could complete the enclosed survey booklet and return it in the enclosed envelope or answer the questions online. A reminder was sent to everyone who had not responded on October 25, 2013. The survey ended on December 31, 2013. The patients were not offered compensation for participation.
Questionnaires and additional questions
The survey booklet/website had 120 questions. The following six validated questionnaires were used in the survey: 1) Short Form 36 Health Survey (SF-36) version 2.0,46 2) Brief Fatigue Inventory (BFI),47 3) Multidimensional Fatigue Inventory 20 (MFI-20),48 4) Hospital Anxiety and Depression Scale (HADS),49 5) European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) version 3.0,50 and 6) Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF).18 For all questionnaires, the required permissions for use in the MPNhealthSurvey were obtained. The questionnaires were available in Danish and validated, with exception of the MPN-SAF questionnaire. The MPN-SAF questionnaire was available in English and validated. This questionnaire was translated into Danish according to guidelines arranged by the research group prior to this survey.51
SF-36 is a generic questionnaire that investigates physical and mental health in addition to physical, mental, and social functioning.46 The questionnaire has 36 items that can be combined into eight scales and two summary measures: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, mental health, a physical component summary measure, and a mental component summary measure. Role-physical measures limitations due to physical health and role-emotional measures limitations due to emotional health, including limitations in daily work or other daily activities and limitations in pursuing hobbies or other leisure activities. The physical component summary measure and the mental component summary measure are derived and scored using a component analysis and takes into account all of the physical or mental components in the questionnaire. The scores and summary measures range from 0 to 100. Higher scores or summary measures indicate the more favorable conditions.
BFI and MFI-20 are symptom-specific fatigue questionnaires.47,48 BFI has nine items that cover the severity of fatigue and the impact on daily functioning. A score for each item can range from 0 to 10. Higher scores indicate more fatigue. A global fatigue score is found by averaging the scores of all items in the questionnaire. MFI-20 has 20 items and the following five scales: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. A score can range from 4 to 20. Higher scores indicate more fatigue.
HADS is a symptom-specific questionnaire that investigates anxiety and depression.49 HADS has 14 items and two scales: anxiety and depression. A score can range from 0 to 21. A score <8 is assessed as no anxiety/depression, a score of 8–10 is assessed as mild anxiety/depression, a score of 11–14 is assessed as moderate anxiety/depression, and a score of 15–21 is assessed as severe anxiety/depression.
EORTC QLQ C-30 is a cancer disease-specific questionnaire that investigates HRQoL.50 The questionnaire has 30 items that can be combined into five functional scales, three symptom scales, six single items, and global health status/QoL. The functional scales cover physical, role, emotional, social, and cognitive functioning. The symptom scales cover fatigue, pain, and nausea/vomiting. The single items assess dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. The scores can range from 0 to 100. Higher scores on functional scales represent a great/healthy level of functioning. Higher scores on symptom scales represent high level of symptomatology. A high score on the global health/QoL represents a high HRQoL. Danish population-based reference data for EORTC QLQ C-30 will be used in future investigation of potential differences in HRQoL between the MPN subgroups and the general population.52
The MPN-SAF is a disease-specific questionnaire that specifically investigates the symptoms and QoL in MPN patients.18 MPN-SAF is co-administered with BFI. MPN-SAF co-administered with BFI includes a total of 26 symptom items and one QoL item. The items are measured on 0–10 rating scale. High numbers indicate higher level of symptomatology or QoL impairment. The MPN-SAF Total Symptom Score (MPN-SAF TSS), which is an abbreviated symptom score developed from MPN-SAF and BFI, was also used in the survey.19 MPN-SAF TSS has 10 items, and the score ranges from 0 to 100.
A total of 25 items in the questionnaire booklet/on the website covered lifestyle, including physical activity, smoking, and alcohol consumption, in addition to height, weight, social life, regularity in the intake of medication prescribed by a doctor, and if someone helped the respondent to complete the questionnaire booklet or to answer the questions on the website. Furthermore, the respondents were able to write in free text.
Comorbidity and lifestyle
Comorbidity and lifestyle will be taken into account in future investigation of HRQoL in the MPN population. We will investigate, if there are differences in comorbidity and lifestyle between ET, PV, and MF participants and differences in lifestyle between the MPN population and the general population. Furthermore, in future research, PROs will be adjusted for comorbidity and lifestyle as confounders.
The Charlson Comorbidity Index (CCI) was used to assess the comorbidity burden. The CCI is a weighted index that consists of disease clusters that include chronic diseases and cancers.53,54 Information on comorbidities was obtained from the NPR, all diagnosis codes that were recorded between September 4, 2013, and the preceding 5 years were included.43 MPNs were excluded from the CCI because the CCI will be used to adjust for the comorbidity confounders during the future investigation of the differences between patients with different MPN subtypes in PROs that were related to MPNs. The CCI scores were grouped into three categories: 1) no or low comorbidity (score of 0), 2) moderate comorbidity (score of 1–2), and 3) severe comorbidity (score of 3 or higher).
The participants in the MPNhealthSurvey were asked questions on height, weight, and lifestyle. BMI was divided into four categories: 1) <18.5, 2) 18.5–24.9, 3) 25.0–29.9, and 4) ≥30.0. Smoking status was divided into three categories: 1) smoker, 2) ex-smoker, and 3) non-smoker. Alcohol consumption was divided into four categories: 1) ≤7, 2) 8–14, 3) 15–21, and 4) ≥22 units per week. Physical activity in leisure time was divided into four categories: 1) hard training and competitive sports several times per week; 2) training, heavy garden work, or similar ≥4 hours per week; 3) walking, bicycling, light garden work, or similar ≥4 hours per week; and 4) reading, watching TV, or other sedentary activities.
Every few years, the National Institute of Public Health conducts a health and morbidity survey of the general population of Denmark. The lifestyle data from the 2013 survey of the general population will serve as reference data in future investigation of potential differences in lifestyle between the MPN subgroups and the general population.55
Characteristics of the respondents and nonrespondents
The CRS provided information regarding the age, sex, and ethnicity (Danish/Western or non-Western).42,44,45 Statistics Denmark’s registers provided information about living arrangement (living alone or living with someone), education (basic school, upper secondary or vocational school, or higher education), and employment (employed or not employed).56 The distributions of respondents and nonrespondents on MPN subtypes were calculated. The disease duration on September 4, 2013 was established using the registration date for the first MPN diagnosis in the NPR.
Response rate, effect of the reminder, and response mode
The total response rate was calculated based on the respondents who answered at least some items either initially or after receiving a reminder. The numbers of patients who initially responded and who responded after receiving a reminder were calculated. Both patients who did not respond to the request to participate in the survey and the patients who returned a survey booklet without answering any of the questions were registered as nonrespondents. The reasons for nonresponse were given spontaneously by some of the nonrespondents who either initiated a telephone call or returned the question booklet without any answers but provided a note explaining the reason for the nonresponse. Finally, the distributions of respondents who returned the survey booklet and who answered the questions online were determined.
Associations between nonresponse and demographic factors
The demographic factors that were considered for potential influence on the nonresponse were age, sex, living arrangement, education, employment, ethnicity, MPN subtype, and duration of disease.
Statistical analysis
Descriptive statistics were used to present the characteristics of respondents and nonrespondents and the online and paper submitters. Chi square tests were used to test for significant differences in contingency tables for demographic factors, and two sided Student’s t-tests were used to test for significant differences in mean age between the groups. Logistic regressions were used to investigate the possible effects of the interactions between age and all other demographic characteristics, between sex and all other demographic characteristics and between MPN subtype and all other demographic characteristics on nonresponse. A significance level of 0.05 was used. SAS version 9.3 was used for all statistical analyses.
Ethics
The Danish Data Protection Agency approved the survey (SJ-RO-02). In Denmark, register studies and questionnaire studies are exempted from requirement of approval from the Committee on Health Research Ethics (Committee Act section 14, 2).57 Future research using the MPN population and the PRO mainstay from the MPN population may require approval from the Committee on Health Research Ethics, which will then be requisitioned.
During the survey, the recipients of the invitation to participate in the survey had the opportunity to contact the research group by phone and by email. Thus, it was possible for them to ask clarifying questions both regarding the invitation to participate in the survey and the survey questions. Completion of survey was deemed to be agreement of consent from the participants.
Results
Participants
A total of 4,704 individuals registered with MPN diagnoses between 1977 and March 31, 2013, were alive, and fulfilled all of the inclusion criteria (Figure 1). Of these individuals, 408 were protected from inquiry by scientific surveys. Therefore, 4,296 individuals were invited to participate in the survey. Eleven individuals were not found at the postal addresses registered in the CRS. Thus, presumably 4,285 individuals received an invitation letter. We were informed by relatives that eight individuals had died in the days leading up to receipt of the invitation to participate in the survey. Finally, 41 individuals replied that they did not know about having an MPN diagnosis and that they had never consulted a hematologist or a doctor at a medical department at a hospital for diagnostic investigation. Thus, 4,236 individuals with an MPN diagnosis in the Danish National Patient Register formed the survey population.
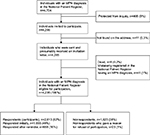  | Figure 1 Flowchart of the survey population. Abbreviation: MPN, myeloproliferative neoplasm. |
Characteristics of the respondents and nonrespondents
The mean age of the nonrespondents was slightly higher than the mean age of the respondents (respondents: age 67.9 years and nonrespondents: age 69.4 years; Table 2). The response rate was slightly higher for males than females, but the difference was not significant (males: 62% and females: 61%). The highest response rate was that of patients aged 70–79 years (females: 67% and males: 68%). The response rate was remarkably higher among those living with someone compared with those living alone (living with someone: 69% and living alone: 51%). The patients with higher education exhibited a higher response rate than the patients who had attended upper secondary/vocational school or basic school (higher education: 71%, upper secondary/vocational school: 67%, and basic school: 52%). The patients who were Danish or living in Denmark but were born in another Western country exhibited a higher response rate than patients who were living in Denmark but were born in a non-Western country (Danish/Western: 63% and non-Western: 44%). The MF patients exhibited the highest response rate compared to the other MPN subgroups (MF: 69%, ET: 60%, PV: 60%, and CML: 66%). The patients who were diagnosed with MPN <1 year before the survey exhibited a higher response rate than those who were diagnosed with MPN≥1 year before the survey, but this difference was not significant (<1 year: 67%, 1–4 years: 63%, and ≥5 years: 61%). Furthermore, the CML respondents had a stronger preference for answering the questions online than the other MPN subgroups, but the difference was not significant (CML online: 24%).
Response rate, effects of the reminder, and response mode
A total of 2,613 (62%) patients responded, including 1,955 (46%) who initially responded and 658 (16%) who responded after receiving a reminder (Figure 1). Of the participants, 2,153 (82%) completed the survey booklet, and 460 (18%) answered the questions online (Table 2).
Associations between nonresponse and demographic factors
An interaction was found between nonresponse, age, and education (p<0.0001), but this was primarily due to the retired participants for whom the amount of missing information regarding education was significant. There were no other interactions between the nonresponses and the demographic factors.
Discussion
A nationwide HRQoL survey of MPN patients (MPNhealthSurvey) was undertaken. In total, 2,613 (62%) patients responded. We believe that the respondents overall covered the broad spectrum of MPN patients, but the highest response rate was that of patients 70–79 years old, living with someone, of a Danish/Western ethnicity, and with a higher level of education. The number of MF participants was only 88. The differences in age, sex, living arrangement, ethnicity, and educational level between respondents and nonrespondents in the Danish National Health Survey performed on the general population in 2010 have been examined, and the pattern of differences is the same as in the MPNhealthSurvey.55
The MPNhealthSurvey has strengths. This was the first nationwide, population-based HRQoL survey of MPN patients, who receive the best available treatment to the extent of its suitability for the patients and if they wish to receive the treatment.17–41 The selection bias was minimized because all MPN patients living in Denmark and registered in the NPR were invited to participate. The mixed-mode approach to data collection ensured that access to and ability to navigate the Internet was not a condition of participating. To the best of our knowledge, this MPN population is the largest ever that submitted PROs.17–41 However, the CML population was larger in, for example, a study by Efficace et al.32,36 Overall, we believe the respondents are representative of the MPN patients in Denmark. However, the differences in characteristics between the respondents and nonrespondents and the relatively small number of MF participants must be taken into consideration when examining the PROs from the respondents. Furthermore, there may be differences in characteristics between respondents and nonrespondents, which we were not aware of. The HRQoL investigation is comprehensive and has not previously been reported on a MPN population to a similar extent.17–41 The differences in PROs between the MPN subgroups were adjusted for age, sex, comorbidity, and lifestyle. The adjustment for confounders was appropriate because we wished to examine HRQoL related to MPN. The CRS register provided data on age and sex, and the NPR provided data on comorbidity. The registers ensured a high validity in the adjustment for the confounders’ age, sex, and comorbidity.
The MPNhealthSurvey has limitations. The NPR is generally considered to be valid, including for hematological malignancies. However, registries are not entirely complete. Because reporting disease diagnoses to the NPR in Denmark is an enforced requirement and is performed systematically, incorrect registrations can only marginally have influenced the results. Individuals who got surprised and frightened when they received an invitation to participate in the survey because they did not know about having an MPN diagnosis registered in the NPR, could contact the research group performing the survey by phone and email. The individuals who turned out not having a connection to a department of hematology or to a department of internal medicine because of MPN, but were registered in the NPR, were recommended to contact their general practitioner to clarify, if the registration in the NPR was a mistake. The large number of questions in the survey may have prevented some patients from participating. The symptomatic patients may have been more motivated to participate than the asymptomatic patients, and the patients with very severe symptom burdens may not have been able to participate, and this may have influenced the PROs. Some MPN patients’ disease coping strategy may include not thinking about the potential long-term effects of the disease. These patients may be nonrespondents in this survey. The survey was based on an anonymous self-report and, for example, unhealthy lifestyles may have been underreported. The survey was based on written questions. Therefore, dyslexic patients, patients with visual impairments, and patients who were not familiar with the Danish language may not have been able to participate or may have had difficulties participating. The questionnaire MPN-SAF has been validated in English. The questionnaire was translated into Danish according to guidelines for translation of questionnaires prior to this survey. However, it has not been fully validated in Danish. It would have been appropriate to fully validate the MPN-SAF in Danish by completing cognitive interviews and thus letting MPN patients validate the translation. Cognitive debriefing of MPN-SAF could potentially have led to appropriate linguistic changes in the questionnaire.51
The reason for not sending an invitation to participate in the survey to patients diagnosed with MPN within half a year prior to the start of the survey was that we believed that newly diagnosed patients could potentially be in psychological crisis because of the recent diagnosis of incurable cancer.
The MPN patients received a reminder, if they had not responded by October 25, 2013. More reminders could have raised the response rate, but because the target population was patients with chronic cancer, more than one reminder could not be justified.
PROs from the MPN patients who developed AML before this survey was performed, and those who underwent bone marrow transplantation before this survey was performed will be separately analyzed.
Conclusion
A nationwide HRQoL survey of MPN patients in Denmark termed MPNhealthSurvey was performed. We believe that the respondents broadly represent the MPN population in Denmark. However, the differences between respondents and nonrespondents have to be taken into consideration when examining PROs from the respondents. The participants provided disease-specific and generic PROs for investigation. The MPNhealthSurvey is the first nationwide and population-based survey, has the largest MPN population, and presents the most extensive PRO collection from a MPN population receiving best available treatment to date. The results of the PRO survey will follow in future articles. The PROs and the MPN population will serve as mainstays for future research. Future research will link to the Danish registries to investigate factors, such as 1) the ability to stay employed, 2) the extent of health service utilization, and 3) mortality.
Acknowledgments
We thank the participants in this survey. Also, we would like to thank Karin Engel Rasmussen for putting together the question booklet and Danish Telemedicine A/S for programming the survey website.
The Einar Willumsen Foundation, the Frimodt-Heineke Foundation, and the Health Science Research Foundation of Region Zealand supported this work.
Author contributions
ADZ, NB, HCH, KJ, EMF, and CLA conceptualized and designed the survey. NB organized the survey, along with AIC and ADZ. EMF provided the statistical analyses. All authors contributed toward data analyses. NB drafted the manuscript. All authors participated in the revision and final approval of the manuscript. All authors agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355(23):2452–2466. | ||
Hultkranz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasm diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995–3001. | ||
Thielen N, Visser O, Ossenkoppele G, Janssen J. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989–2012. Eur J Haemtol. 2016;97(2):145–154. | ||
Barosi G, Rosti V, Bonetti E, et al. Evidence that prefibrotic myelofibrosis is aligned along a clinical and biological continuum featuring primary myelofibrosis. PLoS One. 2012;7(4):e35631. | ||
Hasselbalch HC, Bjørn ME. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediators Inflamm. 2015;2015:102476. | ||
Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340(17):1330–1340. | ||
Kantarjian HM, Dixon D, Keating MJ, et al. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61(7):1441–1446. | ||
Vannucchi AM, Guglielmelli P, Tefferi A. Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin. 2009;59(3):171–191. | ||
Barbui T, Barosi G, Birgegaard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770. | ||
Björkholm M, Ohm L, Eloranta S, et al. Succes story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29(18):2514–2520. | ||
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 Update on diagnosis, therapy, and monitoring. Am J Hematol. 2016;91(2):252–265. | ||
Kiladjian JJ, Giraudier S, Cassinat B. Interferon-alpha for the therapy of myeloproliferative neoplasms: targeting the malignant clone. Leukemia. 2016;30(4):776–781. | ||
Hasselbalch HC, Silver RT. Interferon in polycythemia vera and related neoplasms. Can it become the treatment of choice without a randomized trial? Exp Rev Hematol. 2015;8(4):439–445. | ||
Mesa R, Scherber RM, Geyer HL. Reducing symptom burden in patients with myeloproliferative neoplasms in the era of Janus Kinas Inhibitors. Leuk Lymphoma. 2015;56(7):1989–1999. | ||
Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–1707. | ||
Stein B, Oh ST, Berenzon D, et al. Polycythemia vera: an appraisal of the biology and management 10 years after the discovery of JAK2 V617F. J Clin Oncol. 2015;33(33):3953–3960. | ||
Mesa R, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 patients. Cancer. 2007;109(1):68–76. | ||
Scherber R, Dueck AC, Johansson P, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408. | ||
Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptoms assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. | ||
Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53(3):441–444. | ||
Abelsson J, Andréasson B, Samuelsson J, et al. Patients with polycythemia vera have worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54(10):2226–2230. | ||
Mitra D, Kaye JA, Piecoro LT, et al. Symptom burden and splenomegaly in patients with myelofibrosis in the United States: a retrospective medical record review. Cancer Med. 2013;2(6):889–898. | ||
Siegel FP, Taucher J, Petrides PE. Aquagenic pruritus in polycythaemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88(8):665–669. | ||
Harrison CN, Mesa RA, Kiladjian JJ, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013;162(2):229–239. | ||
Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123(24):3803–3810. | ||
Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and merging treatment options. Ann Hematol. 2014;93(12):1965–1976. | ||
Mesa R, Verstovsek S, Kiladjian JJ, et al. Changes in quality of life and disease-related symptoms in patients with polycythemia vera receiving ruxolitinib or standard therapy. Eur J Haematol. 2016;97(2):192–200. | ||
Anderson LA, James G, Duncombe AS, et al. Myeloproliferative neoplasm patient symptom burden and quality of life: evidence of significant impairment compared to controls. Am J Hematol. 2015;90(10):864–870. | ||
Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer. 2016;122(3):477–485. | ||
Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the Landmark survey. BMC Cancer. 2016;16:167. | ||
Geyer H, Scherber R, Kosiorek H, et al. Symptomatic profiles of patients with polycythemia vera: implications of inadequately controlled disease. J Clin Oncol. 2016;34(2):151–159. | ||
Efficace F, Baccarani M, Breccia M, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554–4560. | ||
Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim DW. Health-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemia. Leuk Res. 2012;36(4):438–442. | ||
Efficace F, Cocks K, Breccia M, et al. Time for a new era in the evaluation of targeted therapies for patients with chronic myeloid leukemia: inclusion of quality of life and other patient-reported outcomes. Crit Rev Oncol Hematol. 2012;81(2):123–135. | ||
Philips KM, Pinilla-Ibarz J, Sotomayor E, et al. Quality of life in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21(4):1097–1103. | ||
Efficace F, Baccarani M, Breccia M, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27(7):1511–1519. | ||
Trask PC, Cella D, Powell C, Reisman A, Whiteley J, Kelly V. Health-related quality of life in chronic myeloid leukemia. Leuk Res. 2013;37(1):9–13. | ||
Guérin A, Chen L, Ionescu-Ittu R, et al. Impact of low-grade adverse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase. Curr Med Res. 2014;30(11):2317–2328. | ||
Mo XD, Jiang Q, Xu LP, et al. Health-related quality of life of patients with newly diagnosed chronic myeloid leukemia treated with allogeneic hematopoietic SCT versus imatinib. Bone Marrow Transplant. 2014;49(4):576–580. | ||
Cella D, Nowinski CJ, Frankfurt O. The impact of symptom burden on patient quality of life in chronic myeloid leukemia. Oncology. 2014;87(3):133–147. | ||
Kekäle M, Peltoniemi M, Aireksinen M. Patient-reported adverse drug reactions and their influence on adherence and quality of life of chronic myeloid leukemia patients on peroral tyrosine kinase inhibitor treatment. Patient Prefer Adherence. 2015;9:1733–1740. | ||
Erlangsen A, Fedyszyn I. Danish nationwide registers for public health and health-related research. Scand J Public Health. 2015;43(4):333–339. | ||
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. | ||
Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(Suppl 7):22–25. | ||
Schmidt M, Pedersen L, Soerensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. | ||
QualityMetrics. Short form 36 health survey. Available from: http://www.sf-36.org/tools/SF36.shtml. Accessed October 7, 2016. | ||
Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. | ||
Smets EMA, Garsson B, Bonke B, De Haes JE. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. | ||
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatr Scand. 1983;67(6):361–370. | ||
European Organisation for Research and Treatment of Cancer. European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Available from: http://groups.eortc.be/qol. Accessed October 7, 2016. | ||
Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8(2):94–104. | ||
Juul T, Petersen MA, Holzner B, Laurberg S, Christensen P, Grønvold M. Danish population-based reference data for the EORTC QLQ C-30: associations with gender, age and morbidity. Qual Life Res. 2014;23(8):2183–2193. | ||
Charlson, M.E, Pompei, P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. | ||
Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. | ||
Christensen AI, Ekholm O, Glümer C, et al. The Danish National Health Survey 2010. Study design and respondent characteristics. Scand J Publ Health. 2012;40(4):391–397. | ||
Statistics Denmark. Available from: www.danmarksstatistik.dk. Accessed October 7, 2016. | ||
The National Committee on Health Research Ethics. Available from: www.dnvk.dk. Accessed October 7, 2016. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.