Back to Journals » Psychology Research and Behavior Management » Volume 10
A narrative literature review of depression following traumatic brain injury: prevalence, impact, and management challenges
Authors Juengst SB, Kumar RG, Wagner AK
Received 22 February 2017
Accepted for publication 9 May 2017
Published 14 June 2017 Volume 2017:10 Pages 175—186
DOI https://doi.org/10.2147/PRBM.S113264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Igor Elman
Shannon B Juengst,1,2 Raj G Kumar,3 Amy K Wagner3–5
1Department of Physical Medicine and Rehabilitation, 2Department of Rehabilitation Counseling, University of Texas Southwestern Medical Center, Dallas, TX, 3Department of Physical Medicine and Rehabilitation, 4Department of Neuroscience, 5Safar Center for Resuscitation Research, University of Pittsburgh, Pittsburgh, PA, USA
Abstract: Depression is one of the most common conditions to emerge after traumatic brain injury (TBI), and despite its potentially serious consequences it remains undertreated. Treatment for post-traumatic depression (PTD) is complicated due to the multifactorial etiology of PTD, ranging from biological pathways to psychosocial adjustment. Identifying the unique, personalized factors contributing to the development of PTD could improve long-term treatment and management for individuals with TBI. The purpose of this narrative literature review was to summarize the prevalence and impact of PTD among those with moderate to severe TBI and to discuss current challenges in its management. Overall, PTD has an estimated point prevalence of 30%, with 50% of individuals with moderate to severe TBI experiencing an episode of PTD in the first year after injury alone. PTD has significant implications for health, leading to more hospitalizations and greater caregiver burden, for participation, reducing rates of return to work and affecting social relationships, and for quality of life. PTD may develop directly or indirectly as a result of biological changes after injury, most notably post-injury inflammation, or through psychological and psychosocial factors, including pre injury personal characteristics and post-injury adjustment to disability. Current evidence for effective treatments is limited, although the strongest evidence supports antidepressants and cognitive behavioral interventions. More personalized approaches to treatment and further research into unique therapy combinations may improve the management of PTD and improve the health, functioning, and quality of life for individuals with TBI.
Keywords: traumatic brain injury, depression, inflammation, antidepressants, rehabilitation
Introduction
Psychiatric disorders, including mood, anxiety, and substance-use disorders, are prevalent after traumatic brain injury (TBI), particularly when the injury is moderate to severe, and can have serious consequences for health, participation, and quality of life. Depression is the most common psychiatric condition to occur post-TBI, although comorbidity rates with other psychiatric and behavioral disorders are high.1,2 Despite the potentially serious consequences of depression, the majority of adults with TBI who experienced depression in the first year post-TBI did not receive treatment.3 Treatment for post-traumatic depression (PTD) is complicated, however, due to its uncertain and multifactorial etiology; that is, it can develop through multiple and multifaceted pathways that may or may not directly result from the primary brain injury.4 Therefore, treatment development for depression must occur in well-defined subgroups having similar symptom clusters or risk factors to address the unique underlying risk factors (eg, neurobiological changes and major life stressors) and tailor treatment accordingly.4 This multifactorial nature of PTD could at least partially explain why response to PTD treatment is often poor compared to the broader major depressive disorder (MDD) population and why PTD treatment studies yield such variable results. This narrative review presents the following: 1) an updated overview of the prevalence and symptom presentation of PTD after moderate to severe TBI; 2) the impact of PTD on other long-term outcomes after moderate to severe TBI; 3) an overview of the various underlying conditions and etiological elements – from neurobiology to life stressors – that contribute to depression post-TBI; and 4) a summary of the successes and challenges in clinical management of PTD.
Methods
For this narrative review,5 defined as a well-structured synthesis of available evidence that conveys a clear message supported by existing data,6 we selected articles through a multistep process. The first author (SBJ) has a web-based literature database that has been continuously updated from 2013 to late 2016 and reflects the author’s research areas of interest, including TBI and depression. Articles included in this database were identified from several sources, including multiple PubMed searches (with weekly updates emailed to and reviewed by the first author to build the database), reference lists of relevant articles, database searches for other TBI-related publications and projects, and recommendations from other experts and collaborators. PubMed searches included the search term “brain injury” AND (alone and in combination): depression, affect, suicide, anxiety, fatigue, cytokine, biomarker, participation, treatment, systematic review. Within this database, we entered “depression” as a search term and reviewed abstracts of all resulting articles for relevance to TBI and depression and categorized articles into one or more of the following headings based on the relevant content: PTD symptoms and prevalence, PTD predictors, PTD impact, PTD treatment, mild TBI, and suicide. Articles published prior to 2000 or not published in English were excluded. Articles were then read to produce overall narrative summaries within each of the identified headings in the “Discussion” section, with priority given to previously published systematic reviews.
Discussion
Prevalence and symptom presentation
Numerous studies have reported prevalence for psychiatric disorders, and specifically, for depression after TBI. Psychiatric diagnoses, including mood, anxiety, and substance abuse disorders, were reported in 75.2% of individuals across the first 5 years post-TBI, with the majority of these (77.7%) emerging in the first year,7 similar to another study indicating clinically significant psychiatric symptoms in 42% of adults with TBI at 6 months post-injury.8 The majority of all psychiatric disorders (56.5%) post-TBI, and two-thirds of PTD diagnoses specifically,9 were first-time diagnoses. Point prevalence rates of psychiatric disorders up to 20 years post-TBI ranged from 17% to 31%,2,10–14 with a systematic review from 2011 estimating a point prevalence of 30% across multiple time points post moderate to severe TBI.15 Cumulative prevalence rates for PTD were higher, just over 50%,3,9,16 indicating that more than half of individuals with TBI will experience a depressive episode after injury. Most individuals will experience their first post-injury major depressive episode within the first year after TBI.3 In addition, individuals with TBI had an elevated risk of suicide attempt (5-fold increase in risk) and completion (3–4 times more likely to die from suicide) compared to the general population.17–19
Depression and TBI share many common somatic symptoms, including fatigue, poor concentration, and sleep disruption. However, a study by Jorge et al20 found that nearly all of the 66 adults with TBI in their cohort with notable depressive symptoms had depressed mood; that is, they found no evidence for “masked” depression. However, they did suggest that the symptoms that best differentiated those with and without depression also differed based on time post-injury, with symptoms related to sleep disruption and poor concentration only distinguishing between groups after 6 months post-injury. Seel et al12 reported that the most common symptoms of depression across the first 10 years post-TBI included fatigue, distractibility, and rumination, and that rumination, self-criticism, and guilt were the symptoms that best differentiated those with depression from those without depression after TBI.21 Furthermore, they found that depression post-TBI was characterized more by irritability, anger, and aggression than by sadness or tearfulness.21
The impact of depression after TBI
PTD has implications for: 1) health, including higher re-hospitalization rates, greater suicide risk, and more caregiver burden; 2) participation, including return to work or school and social relationships; and 3) quality of life, including life satisfaction and overall well-being. Emotional changes, in particular, influenced long-term functional, psychosocial, and quality of life, especially as time since injury increased.2,3,22–28 Psychiatric conditions, such as depression, accounted for a majority of all re-hospitalizations beyond the first year post-injury,29,30 resulting in increased medical costs, and depression was among the strongest contributors to the high suicide risk reported after TBI.19,31 Depression was also significantly associated with poor vocational,32,33 psychosocial,2,32 and functional independence outcomes.32,34 A systematic review conducted by Garrelfs et al33 reported a strong negative association between depression and return to work after acquired brain injury. Furthermore, depression was associated with poorer social functioning after TBI.2,25 One potential pathway through which depression could lead to poor participation outcomes is through disrupted behavior, such as aggression, impulsivity, or poor decision making. Post-TBI depression was highly associated with disrupted behavior after TBI,2,35 with some evidence indicating that depression preceded behavioral disruption,36,37 and disrupted behavior was a significant predictor of poor participation outcomes.38 Often, however, it is difficult to differentiate cause from effect with regard to the relationship between PTD and other TBI-related impairments (Unpublished Material. Kumar RG, Gao S, Juengst S, Wagner AK, Fabio A. Post-traumatic depression effects on cognitive and physical impairments after moderate to severe civilian traumatic brian injury: a thematic review). For example, Perrin et al39 found that functional independence may be more of a cause than a consequence of PTD, though they concluded that there was likely reciprocal causation between the two. Other predictors of PTD are discussed in the “Predictors/etiology of depression” section. Finally, PTD up to and beyond 10 years post-injury was associated with lower and declining life satisfaction40–42 and with poorer quality of life after TBI.3,25,43–45 A study by Diaz et al45 reported that those with depression after TBI had greater impairments in all domains of the Short Form Health Survey (SF-36), a health-related quality of life measure, compared to those without depression.
Predictors/etiology of depression
The presentation of PTD is a multifactorial response to a number of underlying factors associated with TBI rather than just the direct result of a primary pathology.4 Therefore, one of the greatest challenges to managing PTD is identifying its unique etiology, which can differ from person to person. Strakowski et al4 emphasized the importance of identifying underlying risk factors for PTD that should be managed first, prior to addressing depressive symptoms, to maximize the chances for a positive treatment response; this included employing focused treatments in well-defined subgroups. As an example, one subgroup may be those who experience fatigue after TBI. Recent evidence suggests that fatigue is directly caused by TBI and may lead to, rather than result from, PTD.46 Therefore, a treatment approach to PTD among those with primary fatigue may include addressing fatigue first. The first step toward personalized treatment approaches to PTD is to understand the heterogeneity in its risk factors and its underlying causes after TBI.
Biological mechanisms of PTD: inflammatory- induced depression
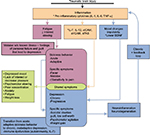
There are numerous biological mechanisms through which depression can develop after TBI,47 including through inflammation (Figure 1). Inflammatory mediators, including cytokines and inflammatory cell surface markers, are elevated with central nervous system (CNS) injury,48,49 including TBI,50,51 and these markers have been linked to depression in neurologically intact adults,52–54 older adults,55 and individuals with chronic conditions associated with inflammation,56–61 including TBI.62,63 Acute cerebral spinal fluid (CSF) inflammation has been shown to be associated with PTD in the first year post-TBI.62 Specifically, higher acute CSF levels of the cell surface markers such as sVCAM-1, sICAM-1, and sFAS were associated with a significant increase in risk for depression 6 months post-TBI, and higher acute CSF levels of IL-12 were associated with PTD at 12 months.62 These markers are known to be associated with inflammation and white matter changes among older adults with depression.55,64 Inflammation occurring early after TBI may contribute to long-term, chronic inflammation even several years post-injury,65 and hence, to chronic risk for depression.
Acute inflammation post-TBI may also lead to depression via maladaptive “sickness behavior” (Figure 1), which shares several symptoms (eg, lack of energy and interest and decreased appetite) with depression.61,66–68 While sickness behavior is initially adaptive and helps the body respond to acute injury and/or infection, it becomes maladaptive if it persists for longer than 3–6 weeks.61,69 This time frame marks a transition from acute, adaptive behavior to a chronic, maladaptive process that may develop into depression.70,71 Cytokines such as IL-6 or tumor necrosis factor-α (TNF-α) are signaling molecules of the immune system that have been associated with MDD.67,72 Elevated serum IL-6, which was significantly elevated up to 3 months after TBI,73,74 is considered a reliable biomarker of MDD,75 though whether it is a biomarker for PTD remains to be determined. While prospective studies are needed to validate this hypothesis, it is reasonable to consider that individuals whose inflammation remains high chronically after TBI may transition from an adaptive immune response (initial high inflammation with return to baseline) to a chronic, maladaptive response (sustained inflammation), leading to PTD and greater disability.
Another cytokine that has shown preliminary associations with PTD is IL-7,62 which has been implicated in lymphoproliferative processes needed to generate an adaptive immune response.76 This connection to generating an effective immune response may explain why low levels of CSF IL-7 in the first week post-injury were inversely related to PTD at 1 year post-injury (eg, levels “below” the 25th percentile in the sample of adults with severe TBI were associated with higher risk for PTD).62 This finding in severe TBI was consistent with a study in MDD in which those in the lowest tertile for serum IL-7 levels had a 3.4-fold greater risk for depression,77 supporting the hypothesis that lower levels of IL-7 among those with depression reflect disruption of T-cell homeostasis as a potential pathway for inflammation-mediated depression.77
IL-6 and TNF-α may also affect brain-derived neurotrophic factor (BDNF) in the setting of chronic stress. Increases in proinflammatory cytokines resulting from chronic stress could potentially lead to dampening of BDNF expression, as evidenced in preclinical models,78 which may then contribute to depression.79 BDNF levels were decreased in untreated depression, but increased with antidepressant treatment.80–82 Notably, in post-stroke depression, serum BDNF levels were higher during periods with no depressive symptoms, but lower in the presence of depressive symptoms.83 BDNF levels in serum during the first week post-TBI may be indicative of risk for the development of PTD by 1 year.84 Overall, BDNF represents a potential biomarker for risk stratification and a potential therapeutic target for both prevention and treatment of PTD.
Psychosocial risk factors for PTD
Pre-injury and comorbid personal factors, as well as changes in functional abilities and community-based participation after injury, could contribute to an adjustment-based, rather than biologically based, depression after TBI.47,85 Adjustment-based depression is characterized more by low self-worth, feelings of guilt, agitation, and suicidal endorsement when compared to depression resulting from maladaptive sickness behavior,69,70 suggesting not only distinct mechanisms for the development of PTD (Figure 1) but also potentially different subtypes of PTD. This point has implications for treatment decisions, as particular subtypes of PTD, such as inflammatory-induced versus adjustment-based, may be more or less responsive to pharmacological versus behavioral interventions.
Age,8,15 race,8,86 less independence in functional tasks after injury,11,15,87–89 engaging in maladaptive coping,90,91 and sleep disturbance or fatigue46,92,93 were all associated with PTD. Furthermore, being unemployed or impoverished at the time of injury or substance abuse before or at the time of injury also conferred a higher likelihood of developing PTD.8,12,13,15,94 Personality characteristics and pre-injury psychiatric conditions that increase the risk of sustaining a TBI95,96 may also put individuals at greater risk for developing PTD.7,85,94 In fact, one of the biggest predictors of PTD was a pre-injury history of depression or other psychiatric disorder.7,94,97 However, the extent to which pre-injury mental health conditions contribute to PTD development through biological versus psychological pathways is unknown.
The appraisal of post-injury abilities compared to the appraisal of pre-injury abilities was another underlying factor related directly to the adjustment to disability that contributes significantly to the development of psychiatric disorders after TBI.87,98–101 Individuals with TBI often over-generalize the effects their injuries have on their abilities and daily lives.102,103 These perceptions were strongly influenced by the individual’s ability or failure to attain post-injury goals.104,105 Individuals with cognitive impairment may have difficulty engaging in goal-directed, problem-solving behavior,106–108 resulting in poor goal attainment and contributing to PTD. Individuals with TBI engaged less frequently in goal-directed problem-solving than individuals without injuries,109 often as a result of associated impairments in executive functions.90,110 Individuals who do engage in goal-directed problem-solving post-injury had higher self-efficacy, less perceived stress, less depression and anxiety, and overall better psychosocial functioning than those who engage in wishful thinking or avoidance behaviors after injury.90,102,109,111 Therefore, goal-directed and problem-solving behaviors may represent one effective avenue of treatment for PTD.
Management challenges
A number of systematic reviews and meta-analyses have been conducted to examine the evidence for efficacious interventions to address PTD, with very limited evidence supporting any approach.27,101,112–118 In addition to (and perhaps partly as a result of) the lack of clear recommendations and guidelines for treating PTD, studies have suggested that a relatively high proportion of individuals living in the community received limited or no treatment for PTD.3,119 Given the long-term consequences of PTD, research to establish evidence-based rehabilitation interventions for depression should be a high priority in rehabilitation research.120–122 Increasing priority should also be given to investigating personalized treatment approaches based on factors ranging from genetic variation to mental health history. Rehabilomics is a rehabilitation-centered conceptual framework that adapts the World Health Organization’s International Classification of Function, Disability, and Health to outline a personalized biopsychosocial approach to rehabilitation research and care,123–126 and it could serve as a contemporary framework from which to conduct innovative and effective research into personalized risk assessment and personalized rehabilitation interventions, where treatment assignment is delineated based on personal biology and other individual factors hypothesized to contribute to PTD.
Current evidence for effective treatment for PTD
The current literature does not support any gold standard for PTD treatment. Antidepressant treatment – specifically serotonergic drugs – and psychotherapeutic approaches – particularly cognitive behavioral approaches – have the best levels of evidence. To date, however, studies on effective treatments for PTD have not differentiated potential subgroups that may have differential responses to treatment.
Pharmacological interventions: Antidepressants have yielded mixed results in studies of depression treatment after neurological injury, such as TBI,112,113 and there is growing evidence that some antidepressants may be less effective among individuals with TBI compared to those without TBI.113 The reduced treatment effectiveness of selective serotonin reuptake inhibitors (SSRIs) after TBI may be due to the high levels of inflammation post-injury. An animal model of postepilepsy depression, another condition associated with high levels of inflammation, revealed that treatment with fluoxetine alone yielded no antidepressant effects, but when paired with an IL-1Ra (an IL-1 antagonist), nearly all depressive indicators were eradicated.127 Currently, treatment with SSRIs is still one of the most evidence-based approaches to PTD treatment,27,113,128 and recent evidence suggested that one such antidepressant (sertraline) may be a promising preventative treatment for PTD.129 Combination therapy with an SSRI and anti-inflammatory agent is also promising, and further study is warranted. However, it is important to note that side effects of newer-generation antidepressant drugs often overlap with and may compound symptoms after TBI.130 Thus, careful consideration should be given to the relative benefits and risks of using these pharmacological agents, particularly where little is known about how they will interact with other TBI-related medical sequelae to cause adverse side effects.
Behavioral/psychological interventions: Cognitive behavioral interventions also have a growing body of evidence to support their efficacy for treating PTD,131–136 though to what extent any specific approach (eg, cognitive behavioral therapy27,134,135 and problem-solving treatments137) was better than another or better than traditional psychotherapy (eg, talk therapy and psychodynamic therapy) still remains to be determined.117,131 Isolating the “active ingredients” of behavioral interventions, particularly if different components are more or less effective for specific subtypes or subgroups of individuals with PTD, will inform clinical triage, training for practitioners, and systems of care.
One meta-analysis suggested that behavioral activation approaches, specifically activity scheduling, showed promise and could be integrated into holistic treatment programs to improve mood in addition to functional and community-based outcomes.138 Problem-solving characterized another class of behavioral intervention that has the potential to improve mood, in addition to other rehabilitation-relevant outcomes such as goal attainment and executive function.27,114 Setting and attaining goals are critical components of the rehabilitation process and may be compromised by executive impairment after TBI.139–141 If goal attainment after TBI is compromised, it may further contribute to the discrepancy between the appraisal of one’s pre- and post-injury abilities, leading to depression. Therefore, interventions that promote goal attainment and address impairments in executive functions may prevent or improve PTD,34,135 particularly if it has more of an adjustment-based etiology.
A review of the literature conducted by the Brain Injury Special Interest Group of the American Congress of Rehabilitation Medicine (ACRM BI-ISIG) recommended metacognitive strategy training, a problem-solving-based approach, as a treatment guideline for executive dysfunction, which often manifests in disrupted behavior, after TBI.142 Impairment in problem-solving mechanisms was at the core of executive dysfunction, and problem-solving was both supported and impeded by emotion after TBI.143 The ACRM BI-ISIG recommended incorporating training in formal problem-solving strategies applied to everyday activities as a cornerstone of cognitive rehabilitation.144 It was also recommended that treatment should be provided in a top-down manner, that is to focus on functional and activity-based goals rather than improvement of symptoms or bodily functions, to ensure generalization to non-trained goals and activities.143 While these strategy-training, self-management approaches are a recommended guideline for treating executive impairments after TBI, the extent to which they improve PTD and its impact on other outcomes remains unclear. Huckans et al145 conducted a pilot study examining a 6- to 8-week group-based cognitive strategy training program for veterans with a history of TBI and found increased life satisfaction and decreased depressive symptoms and cognitive complaints among those who completed the intervention. However, despite evidence that combining cognitive and emotional interventions yielded more positive outcomes than addressing cognition and emotion in isolation,146,147 there is little research assessing the direct effect of problem-solving approaches on PTD.
Other potential pharmacological and non-pharmacological approaches to effectively treat PTD have shown some promise, but require further study. As mentioned earlier, targeting inflammation and/or BDNF levels may help to improve depressive symptoms or prevent the transition from adaptive to maladaptive sickness behavior. There is emerging evidence for agents that selectively enhance BDNF improving depressive symptoms in MDD.115 Exercise-based interventions148,149 may also improve depressive symptoms partially by enhancing BDNF. Targeting inflammation through transcranial photobiomodulation150 was found to improve mood in MDD and through an anti-inflammatory diet58 was found to improve mood in spinal cord injury. Other potential avenues for research that may reveal effective treatments for PTD include methylphenidate118,151 and mindfulness-based interventions.152,153 Recommendations for non-pharmacological treatments of PTD, based on evidence from a systematic review conducted by the French Society of Physical Medicine and Rehabilitation, included structured holistic approaches and family and systemic therapies, though the authors note that this was based on low levels of evidence.136 Finally, further research is required to assess the efficacy of interventions employed in clinical practice that demonstrate anecdotal success for treating PTD, but lack a sufficient evidence base.
Challenges and considerations
In addition to a lack of clear PTD treatment guidelines, there are other challenges and considerations when managing PTD. During the initial hospitalization after TBI, 95% of patients were prescribed a psychotropic medication, with over half of the medications classified as antidepressants.154 Many of these antidepressants, however, were used more for sedation (eg, mirtazapine and trazodone) than for depression.155 Often reasons for prescribing antidepressants in the hospital are not well documented, and patients with TBI may continue to take these antidepressants after discharge from the hospital without a clear indication as to the reason for continued use. Further, while an antidepressant may be effective in addressing other symptoms, the drug class and/or dose may not be effective in reducing depressive symptoms. Polypharmacy is also a significant concern, as individuals with TBI are prescribed multiple medications for several conditions or symptoms. In a large study of adults admitted to inpatient rehabilitation after TBI, 31.8% of patients were receiving ≥6 psychotropic medications. Similarly, a study on polypharmacy in veterans indicated that those with comorbid TBI, anxiety, and mood disorders had the highest odds (adjusted odds ratios [AOR] 15.30) of psychotropic polypharmacy (≥5 medications), and after controlling for these comorbid conditions, polypharmacy was associated with greater risk of overdose and suicidality.156 Polypharmacy is also a problem when combining psychotropic medications with medications for other active medical conditions, such as preexisting chronic disease or other secondary conditions post-TBI (eg, seizures and migraine). Particular consideration should be given to drug interactions that may increase the risk of intracranial bleeds, such as that associated with combined use of antidepressants and nonsteroid anti-inflammatory drugs in the general population,157 though no studies have investigated this interaction after TBI specifically. Physicians may be reluctant to prescribe antidepressants in these cases due to risk for unwanted side effects, especially if an individual had previously not responded to an initial depression treatment.
Individuals with TBI experience many barriers to care, which may be particularly compounded when seeking care for PTD. A study by Matarazzo et al158 that solicited feedback from clinical providers treating veterans with a history of TBI and comorbid mental health conditions presented an illustrative example of these compounded barriers. The clinician-perceived barriers preventing veterans with TBI from seeking care included a lack of knowledge of where to go for treatment, cost of treatment, embarrassment or concern about how they would be perceived by others, and fear of it having a negative impact on their careers. Traditional mental health practitioners may not understand or know how to manage behaviors or unique emotional regulation difficulties of individuals with TBI; similarly, community-based physicians and other medical providers may not feel equipped to treat PTD or may not be aware of the unique considerations (eg, polypharmacy, contraindicated medications after TBI, and differential response to antidepressants) required when considering how to treat adults with TBI. In addition, some interventions may require modifications,132,135 such as pacing or presenting information in different formats, and clinicians may not know how to appropriately adapt these interventions for adults with TBI.158
Other clinical considerations for PTD management include: 1) cultural values, both with regard to how they contribute to depression and how they impact perceptions about treatment;159 2) pain, which co-occurs frequently with depression160 and should be managed as part of prevention and treatment of mental health conditions;114 3) personalized risk and treatment response, based on personal biology and/or personal genetics,161,162 an emerging field that requires further study.
The evidence to support PTD treatment continues to grow, with multiple avenues to explore further. However, even the most evidence-based treatments are only effective for certain individuals. Overall, no single PTD treatment is likely to be universally effective and, therefore, personalized approaches to the research and management of PTD are necessary. The Rehabilomics framework is one tool to operationalize the multifactorial nature of PTD and provides the necessary theoretical groundwork to advance our understanding and management of PTD in the emerging practice of personalized medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
Hibbard MR, Uysal S, Kepler K, Bogdany J, Silver J. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13(4):24–39. | ||
Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61(1):42–50. | ||
Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303(19):1938–1945. | ||
Strakowski SM, Adler CM, Delbello MP. Is depression simply a nonspecific response to brain injury? Curr Psychiatry Rep. 2013;15(9):386. | ||
Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006;5(3):101–117. | ||
Liumbruno GM, Velati C, Pasqualetti P, Franchini M. How to write a scientific manuscript for publication. Blood Transfus. 2013;11(2):217–226. | ||
Alway Y, Gould KR, Johnston L, McKenzie D, Ponsford J. A prospective examination of Axis I psychiatric disorders in the first 5 years following moderate to severe traumatic brain injury. Psychol Med. 2016;46(6):1331–1341. | ||
Hart T, Benn EKT, Bagiella E, et al. Early trajectory of psychiatric symptoms after traumatic brain injury: relationship to patient and injury characteristics. J Neurotrauma. 2014;31(7):610–617. | ||
Whelan-Goodinson R, Ponsford J, Johnston L, Grant F. Psychiatric disorders following traumatic brain injury: their nature and frequency. J Head Trauma Rehabil. 2009;24(5):324–332. | ||
Jourdan C, Bayen E, Pradat-Diehl P, et al. A comprehensive picture of 4-year outcome of severe brain injuries. Results from the PariS-TBI study. Ann Phys Rehabil Med. 2016;59(2):100–106. | ||
Hart T, Hoffman JM, Pretz C, Kennedy R, Clark AN, Brenner LA. A longitudinal study of major and minor depression following traumatic brain injury. Arch Phys Med Rehabil. 2012;93(8):1343–1349. | ||
Seel RT, Kreutzer JS, Rosenthal M, Hammond FM, Corrigan JD, Black K. Depression after traumatic brain injury: a National Institute on Disability and Rehabilitation Research Model Systems multicenter investigation. Arch Phys Med Rehabil. 2003;84(2):177–184. | ||
Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2003;84(10):1449–1457. | ||
Fisher LB, Pedrelli P, Iverson GL, et al. Prevalence of suicidal behaviour following traumatic brain injury: longitudinal follow-up data from the NIDRR Traumatic Brain Injury Model Systems. Brain Inj. 2016;30(11):1311–1318. | ||
Guillamondegui OD, Montgomery SA, Phibbs FT, et al. Traumatic Brain Injury and Depression. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK62061/. Accessed June 30, 2014. | ||
Glenn MB, O’Neil-Pirozzi T, Goldstein R, Burke D, Jacob L. Depression amongst outpatients with traumatic brain injury. Brain Inj. 2001;15(9):811–818. | ||
Harrison-Felix CL, Whiteneck GG, Jha A, DeVivo MJ, Hammond FM, Hart DM. Mortality over four decades after traumatic brain injury rehabilitation: a retrospective cohort study. Arch Phys Med Rehabil. 2009;90(9):1506–1513. | ||
Simpson G, Tate R. Suicidality in people surviving a traumatic brain injury: prevalence, risk factors and implications for clinical management. Brain Inj. 2007;21(13–14):1335–1351. | ||
Reeves RR, Laizer JT. Traumatic brain injury and suicide. J Psychosoc Nurs Ment Health Serv. 2012;50(3):32–38. | ||
Jorge RE, Robinson RG, Arndt S. Are there symptoms that are specific for depressed mood in patients with traumatic brain injury? J Nerv Ment Dis. 1993;181(2):91–99. | ||
Seel RT, Macciocchi S, Kreutzer JS. Clinical considerations for the diagnosis of major depression after moderate to severe TBI. J Head Trauma Rehabil. 2010;25(2):99–112. | ||
Kashluba S, Hanks RA, Casey JE, Millis SR. Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):904–911. | ||
Fann JR, Katon WJ, Uomoto JM, Esselman PC. Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. Am J Psychiatry. 1995;152(10):1493–1499. | ||
Pagulayan KF, Hoffman JM, Temkin NR, Machamer JE, Dikmen SS. Functional limitations and depression after traumatic brain injury: examination of the temporal relationship. Arch Phys Med Rehabil. 2008;89(10):1887–1892. | ||
Hibbard MR, Ashman TA, Spielman LA, Chun D, Charatz HJ, Melvin S. Relationship between depression and psychosocial functioning after traumatic brain injury. Arch Phys Med Rehabil. 2004;85 (4 suppl 2):S43–S53. | ||
Bowen A, Neumann V, Conner M, Tennant A, Chamberlain MA. Mood disorders following traumatic brain injury: identifying the extent of the problem and the people at risk. Brain Inj. 1998;12(3):177–190. | ||
Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma. 2009;26(12):2383–2402. | ||
Lin M-R, Chiu W-T, Chen Y-J, Yu W-Y, Huang S-J, Tsai M-D. Longitudinal changes in the health-related quality of life during the first year after traumatic brain injury. Arch Phys Med Rehabil. 2010;91(3):474–480. | ||
Ifu DX, Kreutzer JS, Marwitz JH, et al. Etiology and incidence of rehospitalization after traumatic brain injury: a multicenter analysis. Arch Phys Med Rehabil. 1999;80(1):85–90. | ||
Marwitz JH, Cifu DX, Englander J, High WM Jr. A multi-center analysis of rehospitalizations five years after brain injury. J Head Trauma Rehabil. 2001;16(4):307–317. | ||
Wasserman L, Shaw T, Vu M, Ko C, Bollegala D, Bhalerao S. An overview of traumatic brain injury and suicide. Brain Inj. 2008;22(11):811–819. | ||
Whelan-Goodinson R, Ponsford J, Schönberger M. Association between psychiatric state and outcome following traumatic brain injury. J Rehabil Med. 2008;40(10):850–857. | ||
Garrelfs SF, Donker-Cools BHPM, Wind H, Frings-Dresen MHW. Return-to-work in patients with acquired brain injury and psychiatric disorders as a comorbidity: a systematic review. Brain Inj. 2015;29(5):550–557. | ||
Hudak AM, Hynan LS, Harper CR, Diaz-Arrastia R. Association of depressive symptoms with functional outcome after traumatic brain injury. J Head Trauma Rehabil. 2012;27(2):87–98. | ||
Juengst SB, Switzer G, Oh BM, Arenth PM, Wagner AK. Conceptual model and cluster analysis of behavioral symptoms in two cohorts of adults with traumatic brain injuries. J Clin Exp Neuropsychol. 2017;39(6):513–524. | ||
Myrga JM, Juengst SB, Failla MD, et al. COMT and ANKK1 genetics interact with depression to influence behavior following severe TBI: an initial assessment. Neurorehabil Neural Repair. 2016;30(10):920–930. | ||
Juengst SB, Myrga JM, Fann JR, Wagner AK. Cross-lagged panel analysis of depression and behavioral dysfunction in the first year after moderate-to-severe traumatic brain injury. J Neuropsychiatry Clin Neurosci. Epub 2017 Mar 15. | ||
Rao V, Lyketsos C. Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics. 2000;41(2):95–103. | ||
Perrin PB, Stevens LF, Sutter M, et al. Reciprocal causation between functional independence and mental health 1 and 2 years after traumatic brain injury: a cross-lagged panel structural equation model. Am J Phys Med Rehabil. | ||
Wood RL, Rutterford NA. Demographic and cognitive predictors of long-term psychosocial outcome following traumatic brain injury. J Int Neuropsychol Soc. 2006;12(3):350–358. | ||
Underhill AT, Lobello SG, Stroud TP, Terry KS, Devivo MJ, Fine PR. Depression and life satisfaction in patients with traumatic brain injury: a longitudinal study. Brain Inj. 2003;17(11):973–982. | ||
Juengst SB, Adams LM, Bogner JA, et al. Trajectories of life satisfaction after traumatic brain injury: influence of life roles, age, cognitive disability, and depressive symptoms. Rehabil Psychol. 2015;60(4):353–364. | ||
Jorge RE. Neuropsychiatric consequences of traumatic brain injury: a review of recent findings. Curr Opin Psychiatry. 2005;18(3):289–299. | ||
Williamson MLC, Elliott TR, Berry JW, Underhill AT, Stavrinos D, Fine PR. Predictors of health-related quality-of-life following traumatic brain injury. Brain Inj. 2013;27(9):992–999. | ||
Diaz AP, Schwarzbold ML, Thais ME, et al. Psychiatric disorders and health-related quality of life after severe traumatic brain injury: a prospective study. J Neurotrauma. 2012;29(6):1029–1037. | ||
Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427–431. | ||
Zgaljardic DJ, Seale GS, Schaefer LS, Temple RO, Foreman J, Elliott TR. Psychiatric disease and post-acute traumatic brain injury. J Neurotrauma. 2015;32(23):1911–1925. | ||
Easton AS. Neutrophils and stroke – can neutrophils mitigate disease in the central nervous system? Int Immunopharmacol. 2013;17(4):1218–1225. | ||
Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurother J Am Soc Exp Neurother. 2010;7(4):366–377. | ||
Pleines UE, Stover JF, Kossmann T, Trentz O, Morganti-Kossmann MC. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J Neurotrauma. 1998;15(6):399–409. | ||
Uzan M, Erman H, Tanriverdi T, Sanus GZ, Kafadar A, Uzun H. Evaluation of apoptosis in cerebrospinal fluid of patients with severe head injury. Acta Neurochir (Wien). 2006;148(11):1157–1164. discussion. | ||
Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. | ||
Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):201–217. | ||
Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. | ||
Dimopoulos N, Piperi C, Salonicioti A, et al. Elevation of plasma concentration of adhesion molecules in late-life depression. Int J Geriatr Psychiatry. 2006;21(10):965–971. | ||
Patten SB, Williams JVA, Lavorato DH, et al. Patterns of association of chronic medical conditions and major depression. Epidemiol Psychiatr Sci. 1–9. Epub 2016 Oct 27. | ||
Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS. Mapping inflammation onto mood: inflammatory mediators of anhedonia. Neurosci Biobehav Rev. 2016;64:148–166. | ||
Allison DJ, Ditor DS. Targeting inflammation to influence mood following spinal cord injury: a randomized clinical trial. J Neuroinflammation. 2015;12:204. | ||
Fischer A, Otte C, Krieger T, et al. Decreased hydrocortisone sensitivity of T cell function in multiple sclerosis-associated major depression. Psychoneuroendocrinology. 2012;37(10):1712–1718. | ||
Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol Allergy Clin North Am. 2009;29(2):309–320. | ||
Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32(1):7–24. | ||
Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute inflammatory biomarker profiles predict depression risk following moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2015;30(3):207–218. | ||
Devoto C, Arcurio L, Fetta J, et al. Inflammation relates to chronic behavioral and neurological symptoms in military with traumatic brain injuries. Cell Transplant. Epub 2016 Oct 12. | ||
Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O’Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18(1):7–13. | ||
Boles J, Goyal A, Kumar R, Wagner A. Chronic Inflammation after Severe Traumatic Brain Injury: Characterization and Associations with Outcome. Nashville, TN: 2013. | ||
Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963–972. | ||
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. | ||
Myers J. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35(5):802–807. | ||
Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29(2):247–264. | ||
Charlton BG. The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Med Hypotheses. 2000;54(1):126–130. | ||
Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32–47. | ||
Littrell JL. Taking the perspective that a depressive state reflects inflammation: implications for the use of antidepressants. Front Psychol. 2012;3:297. | ||
Kumar RG, Diamond ML, Boles JA, et al. Acute interleukin-6 trajectories after TBI: relationship to isolated injury and polytrauma and associations with outcome. J Neurotrauma. 2014;31(12): A104–A105. | ||
Kumar RG, Boles JA, Wagner AK. Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6- and 12-moths post-injury. J Head Trauma Rehabil. 2015;30(6):369–381. | ||
Mössner R, Mikova O, Koutsilieri E, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8(3):141–174. | ||
Katzman SD, Hoyer KK, Dooms H, et al. Opposing functions of IL-2 and IL-7 in the regulation of immune responses. Cytokine. 2011;56(1):116–121. | ||
Lehto SM, Huotari A, Niskanen L, et al. Serum IL-7 and G-CSF in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):846–851. | ||
You Z, Luo C, Zhang W, et al. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225(1):135–141. | ||
Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. | ||
Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry J-M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. | ||
Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. | ||
Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci. 2010;64(4):341–357. | ||
Yang L, Zhang Z, Sun D, et al. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int J Geriatr Psychiatry. 2011;26(5):495–502. | ||
Failla MD, Juengst SB, Arenth PM, Wagner AK. Preliminary associations between brain-derived neurotrophic factor, memory impairment, functional cognition, and depressive symptoms following severe TBI. Neurorehabil Neural Repair. 2016;30(5):419–430. | ||
Rogers JM, Read CA. Psychiatric comorbidity following traumatic brain injury. Brain Inj. 2007;21(13–14):1321–1333. | ||
Perrin PB, Krch D, Sutter M, et al. Racial/ethnic disparities in mental health over the first two years after traumatic brain injury: a model systems study. Arch Phys Med Rehabil. 2014;95(12):2288–2295. | ||
Malec JF, Brown AW, Moessner AM, Stump TE, Monahan P. A preliminary model for posttraumatic brain injury depression. Arch Phys Med Rehabil. 2010;91(7):1087–1097. | ||
Schönberger M, Ponsford J, Gould KR, Johnston L. The temporal relationship between depression, anxiety, and functional status after traumatic brain injury: a cross-lagged analysis. J Int Neuropsychol Soc. 2011;17(5):781–787. | ||
Jourdan C, Bayen E, Vallat-Azouvi C, et al. Late functional changes post-severe traumatic brain injury are related to community reentry support: results from the PariS-TBI cohort. J Head Trauma Rehabil. Epub 2017 Jan 5. | ||
Anson K, Ponsford J. Coping and emotional adjustment following traumatic brain injury. J Head Trauma Rehabil. 2006;21(3):248–259. | ||
Finset A, Andersson S. Coping strategies in patients with acquired brain injury: relationships between coping, apathy, depression and lesion location. Brain Inj. 2000;14(10):887–905. | ||
Rao V, McCann U, Han D, Bergey A, Smith MT. Does acute TBI-related sleep disturbance predict subsequent neuropsychiatric disturbances? Brain Inj. 2014;28(1):20–26. | ||
Beaulieu-Bonneau S, Ouellet M-C. Fatigue in the first year after traumatic brain injury: course, relationship with injury severity, and correlates. Neuropsychol Rehabil. 1–19. Epub 2016 Apr 1. | ||
Bombardier CH, Hoekstra T, Dikmen S, Fann JR. Depression trajectories during the first year after traumatic brain injury. J Neurotrauma. 2016;33(23):2115–2124. | ||
Vassallo JL, Proctor-Weber Z, Lebowitz BK, Curtiss G, Vanderploeg RD. Psychiatric risk factors for traumatic brain injury. Brain Inj. 2007;21(6):567–573. | ||
Fann JR, Leonetti A, Jaffe K, Katon WJ, Cummings P, Thompson RS. Psychiatric illness and subsequent traumatic brain injury: a case control study. J Neurol Neurosurg Psychiatry. 2002;72(5):615–620. | ||
Kumar RG, Bracken MB, Clark AN, Nick TG, Melguizo MS, Sander AM. Relationship of preinjury depressive symptoms to outcomes 3 mos after complicated and uncomplicated mild traumatic brain injury. Am J Phys Med Rehabil. 2014;93(8):687–702. | ||
Ownsworth T, Fleming J, Haines T, et al. Development of depressive symptoms during early community reintegration after traumatic brain injury. J Int Neuropsychol Soc. 2011;17(1):112–119. | ||
Cantor JB, Ashman TA, Schwartz ME, et al. The role of self-discrepancy theory in understanding post-traumatic brain injury affective disorders: a pilot study. J Head Trauma Rehabil. 2005;20(6):527–543. | ||
Draper K, Ponsford J. Long-term outcome following traumatic brain injury: a comparison of subjective reports by those injured and their relatives. Neuropsychol Rehabil. 2009;19(5):645–661. | ||
Ponsford J, Sloan S, Snow P. Traumatic Brain Injury Rehabilitation for Everyday Adaptive Living. Hove, East Sussex, New York, NY: Psychology Press; 2012. | ||
Moore AD, Stambrook M. Coping strategies and locus of control following traumatic brain injury: relationship to long-term outcome. Brain Inj. 2009. Available from: http://informahealthcare.com/doi/abs/10.3109/02699059209008129. Accessed September 26, 2013. | ||
Moore AD, Stambrook M. Cognitive moderators of outcome following traumatic brain injury: a conceptual model and implications for rehabilitation. Brain Inj. 1995;9(2):109–130. | ||
Bandura A, Locke EA. Negative self-efficacy and goal effects revisited. J Appl Psychol. 2003;88(1):87–99. | ||
Trombly CA, Radomski MV, Trexel C, Burnett-Smith SE. Occupational therapy and achievement of self-identified goals by adults with acquired brain injury: phase II. Am J Occup Ther. 2002;56(5):489–498. | ||
Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogn Psychol. 1996;30(3):257–303. | ||
Levine B, Schweizer TA, O’Connor C, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. | ||
Bergquist TF, Jackets MP. Awareness and goal setting with the traumatically brain injured. Brain Inj. 1993;7(3):275–282. | ||
Tomberg T, Toomela A, Pulver A, Tikk A. Coping strategies, social support, life orientation and health-related quality of life following traumatic brain injury. Brain Inj. 2005;19(14):1181–1190. | ||
Krpan KM, Levine B, Stuss DT, Dawson DR. Executive function and coping at one-year post traumatic brain injury. J Clin Exp Neuropsychol. 2007;29(1):36–46. | ||
Rutterford NA, Wood RL. Evaluating a theory of stress and adjustment when predicting long-term psychosocial outcome after brain injury. J Int Neuropsychol Soc. 2006;12(3):359–367. | ||
Price A, Rayner L, Okon-Rocha E, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2011;82(8):914–923. | ||
Neurobehavioral Guidelines Working Group, Warden DL, Gordon B, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468–1501. | ||
Luauté J, Hamonet J, Pradat-Diehl P, SOFMER. Behavioral and affective disorders after brain injury: French guidelines for prevention and community supports. Ann Phys Rehabil Med. 2016;59(1):68–73. | ||
Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol. 2010;21(5–6):427–437. | ||
Stalder-Lüthy F, Messerli-Bürgy N, Hofer H, Frischknecht E, Znoj H, Barth J. Effect of psychological interventions on depressive symptoms in long-term rehabilitation after an acquired brain injury: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94(7):1386–1397. | ||
Gertler P, Tate RL, Cameron ID. Non-pharmacological interventions for depression in adults and children with traumatic brain injury. Cochrane Database Syst Rev. 2015;12:CD009871. | ||
Vattakatuchery J, Lathif N, Joy J, Cavanna A, Rickards H. Pharmacological interventions for depression in people with traumatic brain injury: systematic review. J Neurol Neurosurg Psychiatry. 2014;85(8):e3. | ||
Vangel SJ Jr, Rapport LJ, Hanks RA, Black KL. Long-term medical care utilization and costs among traumatic brain injury survivors. Am J Phys Med Rehabil. 2005;84(3):153–160. | ||
Rapoport MJ, Mccullagh S, Streiner D, Feinstein A. The clinical significance of major depression following mild traumatic brain injury. Psychosomatics. 2003;44(1):31–37. | ||
Kendall E, Terry D. Predicting emotional well-being following traumatic brain injury: a test of mediated and moderated models. Soc Sci Med. 2009;69(6):947–954. | ||
Gordon WA, Zafonte R, Cicerone K, et al. Traumatic brain injury rehabilitation: state of the science. Am J Phys Med Rehabil. 2006;85(4):343–382. | ||
Wagner AK. TBI translational rehabilitation research in the 21st century: exploring a Rehabilomics research model. Eur J Phys Rehabil Med. 2010;46(4):549–556. | ||
Wagner AK. Rehabilomics: a conceptual framework to drive biologics research. PM R. 2011;3(6 suppl 1):S28–S30. | ||
Wagner AK, Zitelli KT. A rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 2013;20(1):39–48. | ||
Crownover J, Wagner A. Chapter 8 – rehabilomics: protein, genetic, and hormonal biomarkers in TBI. In: Wang K, Zhang Z, Kobeissy F, editors. Biomarkers of Brain Injury & Neurological Disorders. Boca Raton: CRC Press/Taylor & Francis Group Co; 2014:236–273. | ||
Pineda EA, Hensler JG, Sankar R, Shin D, Burke TF, Mazarati AM. Interleukin-1β causes fluoxetine resistance in an animal model of epilepsy-associated depression. Neurotherapeutics. 2012;9(2):477–485. | ||
Plantier D, Luauté J, SOFMER Group. Drugs for behavior disorders after traumatic brain injury: systematic review and expert consensus leading to French recommendations for good practice. Ann Phys Rehabil Med. 2016;59(1):42–57. | ||
Jorge RE, Acion L, Burin DI, Robinson RG. Sertraline for preventing mood disorders following traumatic brain injury: a randomized clinical trial. JAMA Psychiatry. 2016;73(10):1041–1047. | ||
Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–288. | ||
Ashman T, Cantor JB, Tsaousides T, Spielman L, Gordon W. Comparison of cognitive behavioral therapy and supportive psychotherapy for the treatment of depression following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. 2014;29(6):467–478. | ||
Ponsford J, Lee NK, Wong D, et al. Efficacy of motivational interviewing and cognitive behavioral therapy for anxiety and depression symptoms following traumatic brain injury. Psychol Med. 2016;46(5):1079–1090. | ||
Fann JR, Bombardier CH, Vannoy S, et al. Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015;32(1):45–57. | ||
Simpson GK, Tate RL, Whiting DL, Cotter RE. Suicide prevention after traumatic brain injury: a randomized controlled trial of a program for the psychological treatment of hopelessness. J Head Trauma Rehabil. 2011;26(4):290–300. | ||
Arundine A, Bradbury CL, Dupuis K, Dawson DR, Ruttan LA, Green REA. Cognitive behavior therapy after acquired brain injury: maintenance of therapeutic benefits at 6 months posttreatment. J Head Trauma Rehabil. 2012;27(2):104–112. | ||
Wiart L, Luauté J, Stefan A, Plantier D, Hamonet J. Non pharmacological treatments for psychological and behavioural disorders following traumatic brain injury (TBI). A systematic literature review and expert opinion leading to recommendations. Ann Phys Rehabil Med. 2016;59(1):31–41. | ||
Rath JF, Simon D, Langenbahn DM, Sherr RL, Diller L. Group treatment of problem-solving deficits in outpatients with traumatic brain injury: a randomised outcome study. Neuropsychol Rehabil. 2003;13(4):461–488. | ||
Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27(3):318–326. | ||
Hart T, Evans J. Self-regulation and goal theories in brain injury rehabilitation: the journal of head trauma rehabilitation. J Head Trauma Rehabil. 2006;21(2):142–155. | ||
Levack WM, Taylor K, Siegert RJ, Dean SG, McPherson KM, Weatherall M. Is goal planning in rehabilitation effective? A systematic review. Clin Rehabil. 2006;20(9):739–755. | ||
Hofer H, Holtforth MG, Frischknecht E, Znoj HJ. Fostering adjustment to acquired brain injury by psychotherapeutic interventions: a preliminary study. Appl Neuropsychol. 2010;17(1):18–26. | ||
Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92(4):519–530. | ||
Gordon WA, Cantor J, Ashman T, Brown M. Treatment of post-TBI executive dysfunction: application of theory to clinical practice. J Head Trauma Rehabil. 2006;21(2):156–167. | ||
Haskins EC. Cognitive Rehabilitation Manual: Translating Evidence-Based Recommendations into Practice. Reston, VA: ACRM Pub; 2012. | ||
Huckans M, Pavawalla S, Demadura T, et al. A pilot study examining effects of group-based Cognitive Strategy Training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47(1):43–60. | ||
Mateer CA, Sira CS. Cognitive and emotional consequences of TBI: intervention strategies for vocational rehabilitation. NeuroRehabilitation. 2006;21(4):315–326. | ||
Mateer CA, Sira CS, O’Connell ME. Putting Humpty Dumpty together again: the importance of integrating cognitive and emotional interventions. J Head Trauma Rehabil. 2005;20(1):62–75. | ||
Hoffman JM, Bell KR, Powell JM, et al. A randomized controlled trial of exercise to improve mood after traumatic brain injury. PM R. 2010;2(10):911–919. | ||
Wise EK, Hoffman JM, Powell JM, Bombardier CH, Bell KR. Benefits of exercise maintenance after traumatic brain injury. Arch Phys Med Rehabil. 2012;93(8):1319–1323. | ||
Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3(3):031404. | ||
Lee H, Kim S-W, Kim J-M, Shin I-S, Yang S-J, Yoon J-S. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol. 2005;20(2):97–104. | ||
Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, part 1: clinical implications for depression, post-traumatic stress disorder, and anxiety. Mil Med. 2016;181(9):961–968. | ||
Bédard M, Felteau M, Marshall S, et al. Mindfulness-based cognitive therapy: benefits in reducing depression following a traumatic brain injury. Adv Mind Body Med. 2012;26(1):14–20. | ||
Hammond FM, Barrett RS, Shea T, et al. Psychotropic medication use during inpatient rehabilitation for traumatic brain injury. Arch Phys Med Rehabil. 2015;96(8 suppl):S256.e14–S253.e14. | ||
Albrecht JS, Kiptanui Z, Tsang Y, et al. Depression among older adults after traumatic brain injury: a national analysis. Am J Geriatr Psychiatry. 2015;23(6):607–614. | ||
Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan War veterans in VA care 2010-2011. Drugs Real World Outcomes. 2016;3:45–52. | ||
Shin J-Y, Park M-J, Lee SH, et al. Risk of intracranial haemorrhage in antidepressant users with concurrent use of non-steroidal anti-inflammatory drugs: nationwide propensity score matched study. BMJ. 2015;351:h3517. | ||
Matarazzo BB, Signoracci GM, Brenner LA, Olson-Madden JH. Barriers and facilitators in providing community mental health care to returning veterans with a history of traumatic brain injury and co-occurring mental health symptoms. Community Ment Health J. 2016;52(2):158–164. | ||
Roy D, Jayaram G, Vassila A, Keach S, Rao V. Depression after traumatic brain injury: a biopsychosocial cultural perspective. Asian J Psychiatr. 2015;13:56–61. | ||
Sullivan-Singh SJ, Sawyer K, Ehde DM, et al. Comorbidity of pain and depression among persons with traumatic brain injury. Arch Phys Med Rehabil. 2014;95(6):1100–1105. | ||
Failla MD, Burkhardt JN, Miller MA, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. 2013;27(6):696–706. | ||
Lanctôt KL, Rapoport MJ, Chan F, et al. Genetic predictors of response to treatment with citalopram in depression secondary to traumatic brain injury. Brain Inj. 2010;24(7–8):959–969. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.