Back to Journals » International Journal of Nanomedicine » Volume 14
A multifunctional supramolecular vesicle based on complex of cystamine dihydrochloride capped pillar[5]arene and galactose derivative for targeted drug delivery
Authors Lu Y, Hou C, Ren J, Yang K, Chang Y, Pei Y, Dong H , Pei Z
Received 18 October 2018
Accepted for publication 9 February 2019
Published 14 May 2019 Volume 2019:14 Pages 3525—3532
DOI https://doi.org/10.2147/IJN.S191256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Yuchao Lu,* 1,2 Chenxi Hou,* 1 Jingli Ren, 1 Kui Yang, 1 Yincheng Chang, 1 Yuxin Pei, 1 Hai Dong, 3 Zhichao Pei 1
1Shaanxi Key Laboratory of Natural Products & Chemical Biology, College of Chemistry and Pharmacy, Northwest A&F University, Yangling, Shaanxi 712100, People’s Republic of China; 2Analysis Center of College of Science & Technology, Hebei Agricultural University, Huanghua, Hebei 061100, People’s Republic of China; 3School of Chemistry & Chemical Engineering, Huazhong University of Science & Technology, Wuhan, Hubei 430074, People’s Republic of China
*These authors contributed equally to this work
Background: Supramolecular vesicles are a novel class of nanocarriers that have great potential in biomedicine.
Methods: A multifunctional supramolecular vesicle (CAAP5G) based on the complex of CAAP5 and galactose derivative (G) assembled via host-guest interaction was constructed.
Results: Using Human embryonic kidney T (293T) cells as experimental models, the cytotoxic effects of CAAP5G was investigated to 0– 50 μmol/L for 24 h. Notably, the CAAP5G vesicles revealed low-toxicity to 293T cells, it was critical to designing drug nano-carriers. Simultaneously, we have evaluated doxorubicin hydrochloride (DOX)-loaded CAAP5G vesicles anticancer efficiency, where DOX-loaded CAAP5G vesicles and free DOX incubated with Human hepatocellular carcinoma cancer cell (HpeG2 cells) and 293T cells for 24 h, 48 h, 72 h. It turned out that CAAP5G vesicles encapsulated anticancer drug (DOX) could decrease DOX side-effect on 293T cells and increase DOX anticancer efficiency. More importantly, the cysteamine as an adjuvant chemotherapy drug was released from CAAP5G vesicles in HepG2 cells where a higher GSH concentration exists. The adjuvant chemotherapy efficiency was evaluated, where free DOX and DOX-loaded CAAP5G vesicles incubated with DOX-resistance HepG2 cells (HepG2-ADR cells) for 24, 48, 72 h, respectively.
Conclusion: The results revealed that the DOX encapsulated by CAAP5G vesicles could enhance the cytotoxicity of DOX and provide insights for designing advanced nano-carriers toward adjuvant chemotherapies.
Keywords: supramolecular vesicles, cysteamine, responsive, adjuvant chemotherapies, targeted drug delivery
Introduction
Drug resistance of cancer cells and side effects to normal cells are major problems in cancer chemotherapy.1–3 Nano-carriers (NC) are one of the most widely explored drug carriers in biomedicine with its distinctive properties.4,5 Especially, targeted and stimuli-responsive nano-carriers (TSNC) can target cancer cells,6–9 achieve a rapid release in the specific tumor microenvironments, enhance cytotoxicity and reduce adverse effect via new concepts or strategies based on nanotechnology.10–13 Vesicles have been one of the most popular TSNC due to their unique cavity that have the potential to encapsulate and controllably release drugs in response to specific stimuli.14–16 Among them, supramolecular vesicles from amphiphilic supra-molecules via host-guest complexation, especially with targeted and stimuli-responsive units, are very important in the application of biotechnology and biomedicine.17–20 Therefore, the construction of targeted and stimuli-responsive vesicles from multi-functional amphiphilic supra-molecule based on host-guest interaction between macrocyclic host molecule and guest molecule is of particular interest and importance in the progress of stimuli-responsive systems for targeted drug delivery.
Pillar[n]arenes, a new class of macrocyclic host molecule, have received much attention in supramolecular chemistry.21–26 Due to their intrinsic symmetrical and rigid structure, accessible modification and excellent properties in host-guest chemistry, pillar[n]arenes have been widely used to construct various interesting supramolecular vesicles for TSNC.27–31 For example, our group developed two types of multifunctional supramolecular vesicles, by using ferrocene or tryptophan capped pillar[5]arene, which exhibited good responsiveness and significantly enhanced the cytotoxicity of DOX.32,33
The cystamine dihydrochloride (CA), an important pharmaceutical intermediate that contained a disulfide bond (sensitive to GSH) and amino groups (sensitive to pH), was investigated.34–36 Most importantly, CA can produce the drug cysteamine in the presence of GSH for adjuvant chemotherapy.37 Previous studies showed that the cysteamine not only inhibited the formation of gastric38 and mammary tumors39 that were induced chemically or after irradiation,40,41 and also enhanced the cytotoxicity of DOX.42 To the best of our knowledge, CA modified pillararene for constructing a dual-responsive amphiphilic host molecule have not yet been reported. We hypothesized that synthesis of CA capping pillararene would not only lead to a GSH-pH dual responsive amphiphilic host molecule, but also the cysteamine produced by the CA could enhance the cytotoxicity of DOX for adjuvant chemotherapy.
In this study, a CA-capped pillar[5]arene (CAAP5) amphiphilic host molecule was first synthesized, and thereafter the multifunctional supramolecular vesicles (CAAP5G) constructed from CAAP5 and a galactose derivative guest molecule (G) based on host-guest interaction for targeted drug nanocarriers (Scheme 1), where the galactose residue on one end of G can act as the targeting ligand to asialoglycoprotein receptor (ASGP-R) overexpressing HepG2 cells via carbohydrate-protein interactions.43
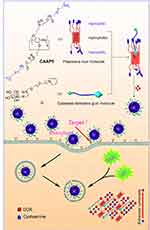 | Scheme 1 Schematic illustration of the synthesis of CAAP5, the host-guest complexation with galactose derivatives (G), formation of vesicles (CAAP5G), and their GSH/pH dual-responsive drug release. |
Material and methods
Instrumentation and materials
All chemical reagents were of analytical reagent grade and used without further purification unless specified. Doxorubicin hydrochloride was purchased from Sangon Biotech. 1NMR spectra were recorded on a Bruker 500 MHz Spectrometer, with working frequencies of 500 MHz for 1H and 125 MHz for 13C nuclei, respectively. Scanning electron microscope (SEM) images were obtained using an S-4800 instrument (Hitachi Ltd.) with an accelerating voltage of 10.0 kV. Negative-stained TEM images were recorded on an H-7650 instrument (Hitachi Ltd. 80 kV). Dynamic light scattering (DLS) measurements were performed on a DelsaTM Nano system (Beckman Coulter, U.S.A.). Water surface tension was recorded with a BZY-3B surface tension measurer (China). An AXIMA-CFR™ plus MALDI-TOF Mass Spectrometer (Kratos, UK) was used for mass analysis. UV-Vis spectra were recorded with a Shimadzu 1750 UV-Visible spectrophotometer (Japan) at 298 K. HepG2 cells and HepG2-ADR cells were obtained from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China). 293T cells were obtained from the KeyGEN BioTECH Co. (Nanjing, China). Cell culture was carried out in an incubator with a humidified atmosphere of 5% CO2 at 37 oC.
Methods
The preparation and characterization of the vesicles
2.46 mg (0.5 µmol) of CAAP5 was dissolved in H2O (0.5 mL) and stirred for 5 min. The solution was mixed 0.5 mL G (1 mM). The final mixture was ultrasonicated for 30 min, then left to stand for 6 h to obtain the vesicles of CAAP5G, which were characterized by SEM, DLS, and TEM, respectively.
DOX loading and release of CAAP5G vesicles
2.46 mg (0.5 µmol) of CAAP5 was dissolved in H2O (0.5 mL) and stirred for 5 min. To the solution were mixed 0.5 mL G (1 µmol) and 0.58 mg DOX (1 µmol). The mixture was ultrasonicated for 30 min and left to stand for 6 h, then subjected to dialysis (molecular weight cutoff 8,000–14,000) in distilled water. DOX-loaded vesicles were obtained when no DOX detected in the water outside the dialysis tube.44
The DOX release from DOX-loaded CAAP5G was studied at different pH buffer solution, with or without GSH (10 mM). 1 mL of DOX-loaded vesicles in a dialysis bag was added into 10 mL of corresponding release medium at room temperature. Within specified time intervals, 0.1 mL of the release medium was taken out for measuring the DOX released with an Epoch microplate spectrophotometer (Biotek).
Cell culture and cell viability
HepG2 cells and HepG2-ADR cells were cultured in RPMI 1640 containing 1% penicillin-streptomycin, 10% FBS, in a humidified atmosphere (5% CO2) at 37 °C. 293T cells were cultured in DMEM medium containing 1% penicillin-streptomycin, 10% FBS, in a humidified atmosphere (5% CO2) at 37 °C. The relative cytotoxicity of unloaded vesicles, DOX and DOX-loaded vesicles were evaluated in vitro by MTT assay, respectively. MTT assay procedures refer to our previous work.44
Results and discussion
To acquire CAAP5, azido-pillar[5]arene H5 and alkynyl cystamine derivative H4 were synthesized according to a published procedure, respectively.45,46 The CAAP5 can be synthesized through Cu-catalyzed azido-alkynyl click reaction. The synthetic details and characterization of CAAP5 and G can be found in the
The host-guest interaction of CAAP5 and G was investigated in D2O via the 1H NMR spectroscopy. The up field chemical shifts of the pyridine protons (H1-3) of G were observed due to the shielding effect of the electron-rich cavities of CAAP5 toward G (see in
To construct the targeting dual-responsive supramolecular vesicles (CAAP5G) based on the CAAP5 and G host-guest interaction, an aqueous solution of CAAP5 and G was sonicated for 30 min. We observed a clear Tyndall effect (Figure 1A right), indicating the existence of abundant nanoparticles, which were further identified by scanning electron microscopy SEM (Figure 1B). TEM image demonstrated the cavity of the vesicles, which was used to loading-drug (Figure 1C). Further analysis with DLS showed that the average diameter of the vesicles was 158 nm (Figure 1D). The critical aggregation concentration (CAC) was found to be 22 µM by the water surface tension method (
 | Figure 1 (A) Tyndall effect of CAAP5G and DOX-loaded CAAP5G; (B) SEM image of CAAP5G vesicles; (C) TEM image of CAAP5G vesicles; (D) DLS histogram of CAAP5G vesicles. |
In the following study, DOX was selected as a model drug to investigate the drug release behavior and encapsulation efficiency of the supramolecular vesicles. As showed in Figure 1A (left), a clear Tyndall effect was observed with DOX-loaded CAAP5G aqueous solution. DLS showed that the average diameter of the DOX-loaded CAAP5G vesicles was 203 nm (
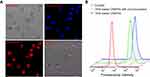
The cellular uptake of DOX-loaded vesicles was investigated by CLSM. Red fluorescence could be observed clearly in the nuclei of HepG2 cells after 4 h incubation with DOX-loaded CAPP5G vesicles, indicating that DOX loaded by CAPP5G vesicles could enter the cancer cells (Figure 3A). To evaluate the targeting effect of CAAP5G vesicles, HepG2 cells were incubated with DOX-loaded CAAP5G vesicles and free DOX at 5 µM for 4 h, respectively, where the efficiency of cellular uptake was analyzed by flow cytometry. HepG2 cells pre-cultured with lactose (2 mg/mL) for 4 h, were used to culture with DOX-loaded CAAP5G vesicles for comparison. As showed in Figure 3B, the fluorescence intensity of HepG2 cells cultured with DOX-loaded CAAP5G vesicles (blue curve) was higher than that of DOX-loaded CAAP5G with lactose pre-incubation (green curve), indicating a higher uptake of DOX-loaded CAAP5G vesicles. The results imply that CAAP5G displays hepatoma-targeting ability. In addition, the cell toxicity of unloaded vesicles to 293T cells are evaluated by the MTT cell survival assay. As indicated in
The anticancer efficency of DOX-loaded CAAP5G vesicles was evaluated using MTT cell survival assay, where HpeG2 cells and 293T cells were incubated with free DOX and DOX-loaded CAAP5G vesicles for 24, 48, and 72 h, respectively. As indicated in Figure 4A, in all three tested periods, the relative viabilities of HepG2 cells after cultured with DOX-loaded CAAP5G vesicles were visibly lower than those with free DOX. By comparison, 293T cells showed higher relative viabilities than those with free DOX (Figure 4A). The results indicated that the encapsulation of DOX by CAAP5G vesicles could enhance the drug anticancer efficiency for HepG2 cells while effectively reduce the side effects to 293T cells.
In the presence of GSH, CAAP5G vesicles can produce the cysteamine, an adjuvant chemotherapy drug, that enhances the cytotoxicity of DOX.42 To study the adjuvant chemotherapy efficiency of CAAP5G vesicles, HepG2-ADR cells were cultured with free DOX and DOX-loaded CAAP5G vesicles for 24, 48, and 72 h, respectively (Figure 5). The results showed that the relative viabilities of HepG2-ADR cells after incubation with DOX-loaded CAAP5G vesicles at all three tested time periods were visibly lower than those with free DOX. It indicated DOX-loaded CAAP5G vesicles could enhance anticancer efficiency of HepG2-ADR cells comparison with free DOX, suggesting CAAP5G as a multifunctional supramolecular vesicle can be used in adjuvant chemotherapy.
Conclusion
In conclusion, novel multifunctional supramolecular vesicles (CAAP5G) based on the host-guest complexation of CAAP5 and G have been successfully constructed and the good drug-loading capability was demonstrated by drug loading experiments. Furthermore, DOX-loaded CAAP5G not only exhibited excellent dual stimuli responsiveness and rapid release of DOX in cancer cells but also possesses targeting ability to ASGP-R overexpressing HepG2 cells. Most notably, the vesicles under high concentration of GSH would release cysteamine, which could enhance the anticancer efficiency of DOX and reduce the drug resistance of cancer cells. This work provides a new idea for rational design in the construction of targeted and stimuli-responsive nanocarriers for drug delivery, which have great applications for adjuvant chemotherapy.
Acknowledgments
This research work was supported by the National Natural Science Foundation of China (No. 21572181 and 21772157). Specialized Research Fund for the Doctoral Program of Hebei Agricultural University (2D201712), and Science & Technology Fund of Hebei Agricultural University (LG201811).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mousa SA, Bharali DJ. Nanotechnology-based detection and targeted therapy in cancer: nano-bioparadigms and applications. Cancers. 2011;3:2888–2903. doi:10.3390/cancers3032888
2. Shao C, Shang K, Xu HB, et al. Facile fabrication of hypericin-entrapped glyconanoparticles for targeted photodynamic therapy. Int J Nanomedicine. 2018;13:4319–4331. doi:10.2147/IJN.S161262
3. Liu J, Huang YR, Kumar A, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710. doi:10.1016/j.biotechadv.2013.11.009
4. Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi:10.1126/science.1095833
5. Zhao ZL, Meng HM, Wang NN, et al. A controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew Chem Int Ed. 2013;52:7487–7491. doi:10.1002/anie.201302557
6. Wang ZH, Tian YF, Zhang H, et al. Using hyaluronic acid-functionalized pH stimuli-responsive mesoporous silica nanoparticles for targeted delivery to CD44-overexpressing cancer cells. Int J Nanomedicine. 2016;11:6485–6497. doi:10.2147/IJN.S117184
7. Cao SP, Pei ZC, Xu YQ, Pei YX. Glyco-nanovesicles with activatable near-Infrared probes for real-time monitoring of drug release and targeted delivery. Chem Mater. 2016;28:4501–4506. doi:10.1021/acs.chemmater.6b01857
8. Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials. 2016;85:152–167. doi:10.1016/j.biomaterials.2016.01.061
9. Zhang Y, Wu XW, Hou CX, et al. Dual-responsive dithio-polydopamine coated porous CeO2 nanorods for targeted and synergistic drug delivery. Int J Nanomedicine. 2018;13:2161–2173. doi:10.2147/IJN.S152002
10. Feng QH, Zhang YY, Zhang WX, et al. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater. 2016;38:129–142. doi:10.1016/j.actbio.2016.04.024
11. Cui W, Li J, Decher G. Self-assembled smart nanocarriers for targeted drug delivery. Adv Mater. 2016;28:1302–1311. doi:10.1002/adma.201502479
12. Dai LL, Liu JJ, Luo Z, Li M, Cai KY. Tumor therapy: targeted drug delivery systems. J Mater Chem B. 2016;4:6758–6772. doi:10.1039/C6TB01743F
13. Liu CQ, Chen ZW, Wang ZZ, et al. A graphitic hollow carbon nitride nanosphere as a novel photochemical internalization agent for targeted and stimuli-responsive cancer therapy. Nanoscale. 2016;8:12570–12578. doi:10.1039/c5nr07719b
14. Meng LB, Zhang WY, Li DQ, et al. pH-responsive supramolecular vesicles assembled by water-soluble pillar[5]arene and a BODIPY photosensitizer for chemo-photodynamic dual therapy. Chem Commun. 2015;51:14381–14384. doi:10.1039/C5CC05785J
15. Gao LY, Zheng B, Chen W, Schalley CA. Enzyme-responsive pillar[5]arene-based polymer-substituted amphiphiles: synthesis, self-assembly in water, and application in controlled drug release. Chem Commun. 2015;51:14901–14904. doi:10.1039/C5CC06207A
16. Chang YC, Yang K, Wei P, et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed. 2014;53:13126–13130. doi:10.1002/anie.201407272
17. Yu GC, Han CY, Zhang ZB, et al. Pillar[6]arene- based photoresponsive host–guest complexation. J Am Chem Soc. 2012;134:8711–8717. doi:10.1021/ja302998q
18. Lee BS, Yip AT, Thach AV, Rodriguez AR, Deming TJ, Kamei DT. The targeted delivery of doxorubicin with transferrin-conjugated block copolypeptide vesicles. Int J Pham. 2015;496:903–911. doi:10.1016/j.ijpharm.2015.10.028
19. Chi XD, Yu GC, Shao L, Chen JZ, Huang FH. A dual-thermoresponsive gemini-type supra-amphiphilic macromolecular [3] pseudorotaxane based on pillar[10]arene/paraquat cooperative complexation. J Am Chem Soc. 2016;138:3168–3174. doi:10.1021/jacs.5b13173
20. Duan QP, Cao Y, Li Y, et al. pH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J Am Chem Soc. 2013;135:10542–10549. doi:10.1021/ja405014r
21. Ogoshi T, Kanai S, Fujinami S, Yamagishi TA, Nakamoto Y. Para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. J Am Chem Soc. 2008;130:5022–5023. doi:10.1021/ja711260m
22. Li H, Chen DX, Sun YL, et al. Viologen-mediated assembly of and sensing with carboxylatopillar[5]arene-modified gold nanoparticles. J Am Chem Soc. 2013;135:1570–1576. doi:10.1021/ja3115168
23. Chen L, Si W, Zhang L, Tang G, Li Z-T, Hou J-L. Chiral selective transmembrane transport of amino acids through artificial channels. J Am Chem Soc. 2013;135:2152–2155. doi:10.1021/ja312704e
24. Yu GC, Jie KC, Huang FH. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem Rev. 2015;115:7240–7303. doi:10.1021/cr5005315
25. Wang YL, Ping GC, Li CJ. Efficient complexation between pillar[5]arenes and neutral guests: from host-guest chemistry to functional materials. Chem Commun. 2016;52:9858–9872. doi:10.1039/C6CC03999E
26. Xiao TX, Zhong WW, Zhou L, et al. Artificial light-harvesting systems fabricated by supramolecular host–guest interactions. Chin Chem Lett. 2019;30:31–36. doi:10.1016/j.cclet.2018.05.034
27. Hu XB, Chen L, Si W, Yu YH, Hou JL. Pillar[5]arene decaamine: synthesis, encapsulation of very long linear diacids and formation of ion pair-stopped [2]rotaxanes. Chem Commun. 2011;47:4694–4696. doi:10.1039/c1cc10633c
28. Wang Y, Xu JF, Chen YZ, et al. Photoresponsive supramolecular self-assembly of monofunctionalized pillar[5]arene based on stiff stilbene. Chem Commun. 2014;50:7001–7003. doi:10.1039/C4CC02760D
29. Chi XD, Ji XF, Xia DY, Huang FH. A dual-responsive supra-amphiphilic polypseudorotaxane constructed from a water-soluble pillar[7]arene and an azobenzene-containing random copolymer. J Am Chem Soc. 2015;137:1440–1443. doi:10.1021/ja512978n
30. Yang K, Pei YX, Wen J, Pei ZC. Recent advances in pillar[n]arenes: synthesis and applications based on host-guest interactions. Chem Commun. 2016;52:9316–9326. doi:10.1039/C6CC03641D
31. Jiang L, Huang X, Chen D, Yan H, Li X, Du X. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar[6]arenes that are responsive to five stimuli. Angew Chem Int Ed. 2017;56:2655–2659. doi:10.1002/anie.201611973
32. Yang K, Chang YC, Wen J, et al. Supramolecular vesicles based on complex of trp-modified pillar[5]arene and galactose derivative for synergistic and targeted drug delivery. Chem Mater. 2016;28:1990–1993. doi:10.1021/acs.chemmater.6b00696
33. Chang YC, Hou CX, Ren JL, et al. Multifunctional supramolecular vesicles based on the complex of ferrocenecarboxylic acid capped pillar[5]arene and a galactose derivative for targeted drug delivery. Chem Commun. 2016;52:9578–9581. doi:10.1039/C6CC03637F
34. Hassan W, Beuzard Y, Rosa J. Inhibition of erythrocyte sickling by cystamine, a thiol reagent. Proc Natl Acad Sci U S A. 1976;73:3288–3292.
35. Aleman MM, Holle LA, Stember KG, et al. Cystamine preparations exhibit anticoagulant activity. PloS One. 2015;10:e0124448. doi:10.1371/journal.pone.0124448
36. Karpuj MV, Becher MW, Springer JE, et al. Prolonged survival and decreased abnormal movements in transgenic model of huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143–149. doi:10.1038/nm0202-143
37. Besouw M, Masereeuw R, Heuvel LVD, Levtchenko E. Cysteamine: an old drug with new potential. Drug Discov Today. 2013;18:785–792. doi:10.1016/j.drudis.2013.02.003
38. Inano H, Onoda M, Suzuki K, Kobayashi H, Wakabayashi K. Inhibitory effects of WR-2721 and cysteamine on tumor initiation in mammary glands of pregnant rats by radiation. Radiat Res. 2000;153:68–74. doi:10.1667/0033-7587(2000)153[0068:ieowac]2.0.co;2
39. Tatsuta M, Iishi H, Yamamura H, Baba M, Mikuni T, Taniguchi H. Inhibitory effect of prolonged administration of cysteamine on experimental carcinogenesis in rat stomach induced by N-methyl-N’-nitro-N-nitrosoguanidine. Int J Cancer. 1988;41:423–426.
40. Fujisawa T, Rubin B, Suzuki A, et al. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PLoS One. 2012;7:e34437. doi:10.1371/journal.pone.0034437
41. Lespinasse F, Oiry J, Fatome M, et al. Radioprotection of EMT6 tumor by a new class of radioprotectors based on a pseudo-peptide cysteamine combination. Int J Radiat Oncol Biol Phys. 1985;11:1035–1038.
42. Wan XM, Zheng F, Zhang L, et al. Autophagy-mediated chemosensitization by cysteamine in cancer cells. Int J Cancer. 2011;129:1087–1095. doi:10.1002/ijc.25771
43. Chen W, Zou Y, Meng FH, et al. Glyco-nanoparticles with sheddable saccharide shells: a unique and potent platform for hepatoma-targeting delivery of anticancer drugs. Biomacromolecules. 2014;15:900–907. doi:10.1021/bm401749t
44. Chang YC, Lv YH, Wei P, et al. Multifunctional glycol-nanofibers: siRNA induced supermolecular assembly for codelivery In vivo. Adv Funct Mater. 2017;27:1703083. doi:10.1002/adfm.201703083
45. Zhang J, Li C, Wang Y, Zhuo RX, Zhang XZ. Controllable exploding microcapsules as drug carriers. Chem Commun. 2011;47:4457–4459. doi:10.1039/c1cc10337g
46. Yang J, Shao L, Yu GC. Construction of pillar[6]arene-based CO2 and UV dual-responsive supra-amphiphile and application in controlled self-assembly. Chem Commun. 2016;52:3211–3214. doi:10.1039/C5CC10617F
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.