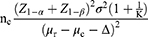Back to Journals » Journal of Pain Research » Volume 15
A Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Study of Jianyao Migu Granules in the Treatment of Osteopenic Low Back Pain
Authors Qin Z, Xu K, Mo W, Ye J, Xu J
Received 5 June 2022
Accepted for publication 23 August 2022
Published 1 September 2022 Volume 2022:15 Pages 2607—2617
DOI https://doi.org/10.2147/JPR.S377082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Zihao Qin, Ke Xu, Wen Mo, Jie Ye, Jinhai Xu
Orthopedics Department, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
Correspondence: Jinhai Xu; Jie Ye, Longhua Hospital Shanghai University of Traditional Chinese Medicine, 725, South Wanping Road, Xuhui District, Shanghai, 200030, People’s Republic of China, Tel +86 18016006692 ; +86 3301880301, Email [email protected]; [email protected]
Purpose: This randomized controlled trial aimed to evaluate the clinical efficacy of Jianyao Migu granules (JYMGG) in the treatment of primary osteopenic low back pain (LBP).
Patients and Methods: A total of 108 patients with primary osteopenic LBP were randomly divided into the JYMGG group and placebo group. Both groups took 600 mg of oral Caltrate D daily; in addition, the JYMGG group was given oral JYMGG, while the placebo group was given placebo granules. The treatment period was 6 months for both groups. The pre- to post-treatment changes in the bone mineral density (BMD), visual analogue scale (VAS) score, Oswestry disability index (ODI), and bone turnover markers were compared between the two groups.
Results: The post-treatment VAS score and ODI were significantly lower than baseline in both groups (P< 0.05). In the JYMGG group, the lumbar BMD increased from 0.88± 0.07 g/cm2 to 0.90± 0.13 g/cm2 and the hip BMD increased from 0.77± 0.08 g/cm2 to 0.78± 0.10 g/cm2, giving increases of 2.70% and 1.96% respectively, but the differences were not statistically significant. The post-treatment levels of ALP, osteocalcin, P1NP, and β-CTX were increased compared with baseline in both groups, but the differences were not statistically significant. The thyrotropin level was significantly increased after treatment in the placebo group (P< 0.05). There were no abnormalities detected in routine blood and kidney function tests performed during the observation period. Some patients showed elevated liver enzymes and gastrointestinal reactions.
Conclusion: JYMGG effectively relieved the bone pain, and improved the quality of life of patients with primary osteopenic LBP.
Keywords: chinese herb, clinical trial, Jianyao Migu granules, osteoporosis
Introduction
Osteoporosis is one of the most common senile diseases worldwide.1 In 2018, the National Health Commission publicly released the results of the first epidemiological survey on osteoporosis in China,2 which showed that the prevalence of low bone mass was 32.9% in Chinese people aged 40–49 years (34.4% among men and 31.4% among women) and 46.4% in those older than 50 years (46.9% among men and 45.9% among women). This low bone mass negatively affects the quality of life of older adults. Furthermore, several cross-sectional studies have shown that patients with osteoporosis more frequently experience low back pain than individuals without osteoporosis.3 A study performed in Japan from 2017 to 2019 found that 58.3% of osteoporotic patients experience pain-related dysfunction.4 The most common symptom of osteoporosis is pain, which is experienced by about 58% of individuals with osteoporosis, among whom 70%–80% have waist pain.5 However, 10.4% of osteoporotic patients with low back pain have no evidence of fracture.6 Although there is still no clear definition of “osteopenic low back pain”, Fujimoto et al defined it as chronic low back pain caused by primary osteoporosis for more than 3 months. The characteristics of osteopenic low back pain are that it usually occurs at rest rather than during exercise and that it is mainly nociceptive rather than neuropathic.7 Osteoporotic fractures caused by long-term chronic pain and limited activity greatly affect the quality of life of patients.8 Therefore, it is important to quickly identify the trend of bone mass reduction in patients with low back pain, focus on osteoporosis prevention and treatment, and perform timely intervention during the period of bone mass reduction.
Although the pathogenesis of osteoporosis is complex, insufficient intake of vitamin D and calcium is an important factor.9 Updated guidelines published in 2020 indicate that adequate intake of calcium and vitamin D is beneficial for achieving ideal peak bone mass, delaying bone mass loss, and improving and maintaining bone health.10 Caltrate D effectively supplements calcium and vitamin D, and is widely used as a basic treatment for osteoporosis in clinical practice. Other treatments for osteoporosis include bone resorption inhibitors, bone formation promoters, functional exercises, and internal and external traditional Chinese medicines.11–13 Traditional Chinese medicine has a long history in the treatment of osteoporosis under the guidance of the “kidney and bone” theory. Jianyao Migu granules (JYMGG) is an empirical prescription for the treatment of osteoporosis created based on the theory of “Focusing on qi and blood first” and many decades of clinical experience by Professor Shi Qi, a master of traditional Chinese medicine in our hospital. JYMGG has been clinically verified to effectively relieve back pain and significantly increase vertebral bone mass.14 Previous animal studies have also shown that JYMGG increases bone mass by increasing the number, thickness, and volume of bone trabeculae.15,16 In the present study, Caltrate D combined with JYMGG was used in the treatment of osteopenic low back pain; the efficacy of JYMGG was objectively evaluated in comparison with Caltrate D combined with placebo as the control.
Materials and Methods
Study Design and Participants
This 6-month multicenter, randomized, double-blind, placebo-controlled clinical study was conducted in three community health service centers: Pengpu Xincun Community in Jing’an District, Shanggang Community in Pudong New Area, and Nanmatou Community in Pudong New Area. We enrolled 108 patients with osteopenic back pain treated between January 2019 and October 2019. The inclusion criteria were: bone mineral density (BMD) between −1 and −2.5, women aged 50 to 75 years and men aged 60 to 75 years, low back pain symptoms (visual analogue scale (VAS) score ≥4), and no other treatment regimen. The exclusion criteria were allergy to the drug components in Caltrate D and JYMGG; secondary osteoporosis or concomitant various endocrine diseases, rheumatic immune diseases, or other serious diseases affecting bone metabolism; specific lumbago caused by tumor, fracture, tuberculosis, or inflammation; patients requiring surgical treatment; patients who could not participate in the study for other reasons; current participation or participation in another study within the last 3 months.
Sample Size Calculation
The main outcome index was the pre- to post-treatment change in the BMD, and the treatment received by the placebo group was considered the basic treatment in the test of superior efficacy. The following sample size calculation formula was used:
where μr was the mean BMD of the JYMGG group, μc was the mean BMD of the placebo group, and σ was the standard deviation of BMD. With α=0.05, β=0.1, Δ=0.025, and K=1 between the JYMGG group and the placebo group, μr=0.866, μc=0.756, and σ=0.125. When the calculated nc=nr=45 and the assumed dropout rate was 20%, 108 cases were needed.
Blinding and Treatment
Patients were randomly assigned to the JYMGG group or the placebo group. The JYMGG group received 600 mg of Caltrate D once daily + one bag of JYMGG twice daily after meals. The JYMGG were composed of Astragali Radix 15 g, Epimedii Folium 12 g, Ecliptae Herba 12 g, Salviae Miltiorrhizae Radix Et Rhizoma 9 g, Sinomenii Caulis 9 g, and Achyranthis Bidentatae Radix 9 g (all the weights mentioned above are the weight of crude herbs, prepared by Sichuan New Green Pharmaceutical Co, Ltd). The placebo group received 600 mg of Caltrate D once daily + one bag of placebo granules twice daily after meals. The placebo granules were 2% caramel pigment, 0.35% tartrazine pigment, 0.04% sunset yellow pigment, and 0.04% bitter agent (sucrose octaacetate) and maltodextrin dissolved in water and sprayed with dry powder to create granules (prepared by Sichuan New Green Pharmaceutical Co, Ltd). All patients were instructed to take the drugs as prescribed for 6 months.
Endpoints
The primary efficacy endpoints were the lumbar and hip BMD (left femoral neck) after 6 months of treatment and the VAS scores after 1, 3, and 6 months of treatment. The secondary efficacy endpoints were the Oswestry Disability Index (ODI) and bone conversion indicators (serum calcium, Thyroid Stimulating Hormone (TSH), 25-hydroxy vitamin D (25-OH-VITD), serum osteocalcin, Procollagen Type 1 N-peptide (P1NP), β-cross-linked C-terminal telopeptide of type I collagen (β-CTX), and serum alkaline phosphatase (ALP)).
Baseline and Follow-Up Measurements
The BMD of the lumbar spine and hip (left femoral neck) were measured at baseline and after 6 months of treatment using a dual-energy X-ray bone density instrument (HologicQDR-2000 DXA). The bone density meter was operated by skilled professional technicians, and the pre- and post-treatment assessments were obtained via the same bone density meter, procedure, and operator.
Lumbar pain and function were assessed using the VAS and ODI, respectively. The VAS was scored by the patients from 0 to 10. A VAS score of 2–4 was taken to indicate mild pain, 5–7 indicated moderate pain, and 8–9 indicated severe pain. The ODI was used to evaluate the waist function by assessing 10 items: the degree of waist and back pain, self-care ability in daily life, ability to lift heavy objects, walking status, sitting status, standing status, sleep status, sexual status, social life status, and travel status. Each item was scored from 0 to 6, with a lower score indicating better function. The VAS score and ODI were measured at baseline, and after 1, 3, and 6 months of treatment.
Bone metabolism was measured at baseline and after 6 months of treatment. The patients began fasting at 8:00 pm on the day before the test. Samples were collected and sent to a third-party testing institution (Kingdomain Testing Company) for measurement on the following morning.
Adverse Events
All patients underwent routine blood tests (white blood cell count, platelet count) and liver and kidney function tests (alanine aminotransferase, aspartate aminotransferase, uric acid, and urea nitrogen) at enrollment and after 6 months of treatment. Any adverse events that occurred during treatment were recorded to evaluate the treatment safety.
Statistical Analyses
SPSS 21.0 for Windows statistical software was used for data processing and statistical analysis. A full analysis set was used for the intention-to-treat analysis, and missing data due to loss of follow-up were supplemented by the last observation data carried forward method. Measurement data were tested for normality of distribution. Data with normal distribution were expressed as mean ± standard deviation. The paired t-test was used for intragroup analyses before and after treatment, the independent sample t-test was used for intergroup analyses, and the Wilcoxon rank sum test was used for non-normally distributed measurement data. The statistical data were described as percentages or constituent ratios, and were analyzed using the χ2 test, Fisher’s exact probability method, or non-parametric testing. With a test level of α=0.05 for two-sided tests, P<0.05 was considered statistically significant. The measurement data of repeated measurement were analyzed by ANOVA, and the Greenhonse-Geisser correction coefficient was used if the covariance matrix did not meet the property of “spherical symmetry”.
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. The mean patient age was 66.13 years. There were no significant differences between the two groups in pre-treatment physical characteristics, including age, height, weight, and body mass index. The two groups had similar baseline lumbar spine and femoral neck BMD values, VAS scores, ODI values, and levels of bone turnover markers.
 |
Table 1 Baseline Characteristics |
Study Withdrawals and Treatment Adherence
A total of 108 eligible patients with primary osteopenic low back pain were included. Among the 54 patients in the treatment group, four dropped out and 50 completed the study. Among the 54 patients in the placebo group, five dropped out and 49 completed the study (Figure 1). In accordance with the study protocol, all dropout cases were analyzed based on the intention-to-treat protocol, with missing data replaced with data from the last follow-up.
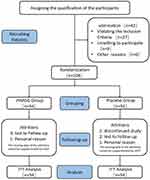 |
Figure 1 Participant flow diagram. Abbreviations: JYMGG, Jianyao Migu granules; LOCF, last observation carried forward; ITT, Intention-to-treat. |
Bone Mineral Density
The BMD data are summarized in Table 2. The lumbar vertebral BMD in the JYMGG group increased from 0.88±0.07 g/cm2 at baseline to 0.90±0.13 g/cm2 after 6 months of treatment, giving an increase of 2.70%; the lumbar vertebral BMD in the placebo group increased from 0.88±0.10 g/cm2 at baseline to 0.90±0.09 g/cm2 after 6 months of treatment, giving an increase of 0.93%. The pre- to post-treatment change in the lumbar vertebral BMD did not significantly differ between the two groups (P>0.05). The femoral neck BMD increased by 1.96% from 0.77±0.08 g/cm2 at baseline to 0.78±0.10 g/cm2 after 6 months of treatment in the JYMGG group and increased by 0.09% from 0.76±0.10 g/cm2 at baseline to 0.75±0.13 g/cm2 after 6 months of treatment in the placebo group. There was no significant difference between groups in the pre- to post-treatment change in the femoral neck BMD (P>0.05).
 |
Table 2 Results for Bone Mineral Density and Bone Turnover Markers |
Visual Analogue Scale Score and Oswestry Disability Index
The changes in the VAS score and ODI are shown in Table 3 and Figure 2. After 3 months and 6 months of treatment, there were significant differences between the two groups in the VAS and ODI (P=0.001 for the VAS and P=0.003 for the ODI after 3 months of treatment; P=0.001 for the VAS and P=0.001 for the ODI after 6 months of treatment). Both groups showed significant differences in the VAS and ODI after 6 months of treatment compared with baseline (P=0.001 for the VAS and P=0.001 for the ODI in the treatment group; P=0.001 for the VAS and P=0.001 for the ODI in the placebo group). Repeated measures ANOVA results showed that the VAS score and ODI in the two groups significantly decreased with the extension of the treatment time (F=102.92, P=0.001 for the VAS score; F=111.92, P=0.001 for the ODI). There were no significant interactions between treatment time and group (F=0.18, P=0.832 for the VAS score; F=0.11, P=0.853 for the ODI), indicating that there was no significant difference between the two groups in the decreases in the VAS score and ODI over time. There were no significant differences between the two groups in the VAS score and ODI after 6 months of treatment (F=0.03, P=0.858 for the VAS score; F=0.13, P=0.720 for the ODI).
 |
Table 3 Results for VAS Score and ODI |
Bone Turnover Markers
The changes in bone metabolism indexes are shown in Table 2. After 6 months of treatment, the ALP, osteocalcin, P1NP, and β-CTX levels tended to be increased compared with baseline in both groups, but these differences were not statistically significant (P>0.05). In the JYMGG group, the TSH level after 6 months of treatment was 23.93% higher than the baseline value (P=0.040) and was significantly higher than the TSH level in the placebo group (P=0.018). In the JYMGG group, the serum calcium and total 25-OH-VITD levels after 6 months of treatment were 4.62% and 18.36% higher than baseline, respectively; however, these increases were not statistically significant (P>0.05). In the placebo group, the serum calcium and total 25-OH-VITD levels after 6 months of treatment were significantly increased by 8.18% and 29.35%, respectively, compared with baseline (P<0.05).
Safety
Adverse events are summarized in Table 4. During the whole treatment process, there were no severe abnormalities in routine blood tests and kidney function tests in either group. The main adverse events were abnormal liver enzyme levels and gastrointestinal reactions. There were three and four patients with abnormal liver enzyme levels in the JYMGG group and the placebo group, respectively; the liver enzyme levels returned to normal 2 weeks after drug withdrawal. Gastrointestinal discomfort, including constipation, heartburn, nausea and vomiting, was reported by 12 patients in the JYMGG group and nine in the placebo group. The overall incidence of adverse reactions did not significantly differ between the two groups (P>0.05).
 |
Table 4 Adverse Events Documented During the 6-Month Trial Period in the ITT Population |
Discussion
This is the first multicenter, randomized, double-blind, placebo-controlled trial to demonstrate the clinical efficacy and safety of a Chinese herbal compound in the treatment of osteopenic low back pain by observing changes in BMD, clinical symptoms, and bone metabolism indicators during a 6-month period. After 6 months of treatment with JYMGG combined with Caltrate D, the VAS score was significantly decreased, and symptoms such as low back pain were effectively improved.
In our study, the lumbar spine and hip BMD were increased after 6 months of treatment in both groups. This is consistent with the results of similar studies. Zhu et al17 reported that in patients treated with herbal formula Xian Ling Gu Bao, the lumbar biphasic BMD response increased by 2.11% in the first 6 months and then decreased by 1.1%, but still remained 1.00% higher than the baseline value. Previous animal studies have shown that JYMGG significantly promotes the proliferation of osteoblasts, promotes the osteogenic differentiation of bone marrow mesenchymal stem cells, stimulates the expression of bone morphogenetic protein-7 and osteocalcin during the differentiation process, and increases the number, thickness, and volume of bone trabeculae to increase bone mass.15,16 Overall, these findings suggest that JYMGG prevents and cures osteoporosis.
We also found that the increase in the hip BMD after 6 months of JYMGG treatment (1.96±13.85%) was lower than the increase in the lumbar spine BMD (2.70±12.01%). This may be because the occurrence of lumbar osteosclerosis increases with age, which interferes with BMD assessments;18 alternatively, it may be because cortical bone in the femoral neck is richer in content than the spine, and its metabolic rate is slow, resulting in later bone loss than trabecular bone.19 Therefore, if only one site can be assessed when evaluating age-related bone loss, the hip may be most representative.20 Although the BMD increased, the difference was not statistically significant either from baseline or from placebo (P>0.05), so the change in bone mass may be dependent on the basic treatment of Caltrate D. However, this is not consistent with the conclusions of our previous animal studies. The reason for this may be that the duration of treatment was shorter than the change cycle of the BMD. Therefore, a longer period of intervention is needed in future studies.
Although JYMGG significantly improves low back pain and lumbar function, the mechanism of pain relief remains unclear. Epimedium is one of the most widely used herbal medicines in traditional anti-osteoporosis herbal prescriptions and is the main component of JYMGG. The anti-osteoporosis effect of JYMGG is partly due to the inhibitory effect of icariin (ICA) and its derivatives on osteoclasts.21 The formation and differentiation of osteoclasts depend on the control of the RANK-RANKL-OPG system. Osteoblasts act on osteoclast precursor cells by expressing RANKL molecules to promote their differentiation and maturation. ICA stimulates the expression of OPG mRNA to make RANKL competitively bound by OPG, thereby inhibiting the formation and activation of osteoclasts.22–24 Although there is no direct evidence that osteoclast activity is related to osteoporotic pain, the association is suggested by the good effect of antiresorptive drugs on osteoporotic pain. Ohtori et al25 reported that patients with osteoporosis without vertebral fractures who received risedronate treatment for 4 months showed a significant improvement in pain and significant decreases in serum and urine levels of N-terminal telopeptide of type I collagen (51.4% and 62.0%, respectively). This suggests that increased osteoclast activity due to osteoporosis may cause pain despite the absence of vertebral fractures.
Another possible analgesic mechanism of JYMGG is that ICA relieves pain by affecting neuropeptides involved in pain transmission (eg, substance P and calcitonin gene-related peptide (CGRP)).26 Substance P, CGRP, and other neuropeptides are widely distributed in the nerve endings around bone marrow and periosteum tissues, which are related to the regulation of local bone conversion, bone injury, inflammation, and cell proliferation, and can affect the microstructure of bone and participate in the occurrence of osteoporosis.27,28 Li et al29 reported that ICA inhibits the continuous production of pain-related factors such as substance P and CGRP by inhibiting the upregulation of cytokine-induced neutrophil chemoattractant-1, thus achieving a more lasting analgesic effect than non-steroidal anti-inflammatory drugs.
The present study found that the TSH levels were significantly higher in the JYMGG group than the placebo group. Population-based observations show that bone mass is correlated with the TSH level30 and TSH receptors are expressed in a large number of osteoblast precursor cells and osteoclasts; thus, TSH regulates osteoblast bone formation and osteoclast bone absorption through different intracellular signals.31,32 TSH promotes the proliferation and differentiation of osteoblasts. Deng et al33 treated rat skull osteoblasts with different doses of TSH and reported that high-dose TSH effectively promotes the proliferation of osteoblasts and increases the expressions of key osteoblast differentiation genes (ALP, BMP2, COL2, and Runx2).
TSH also has a direct inhibitory effect on osteoclast differentiation. Ma et al34 reported that the level of OPG is significantly increased by TSH stimulation in a constructed embryonic stem cell system, partially inhibiting the differentiation of embryonic stem cells into osteoclasts, and the TSH-related inhibition of osteoclast formation is significantly weakened after the addition of OPG antibody. These results support the observation that early TSH negatively regulates osteoclast formation. Furthermore, Agrawal et al35 reported that TSH receptor haploid-deficient mice still showed high turnover of osteoporosis after excluding the influence of thyroid hormone. This suggests that the effect of TSH on bone mass is independent of thyroid hormone. Therefore, we can speculate that JYMGG not only promotes bone formation, but also alleviates bone loss by regulating the TSH level.
After 6 months of treatment, the serum levels of calcium and total 25-OH-VITD were increased in both groups, and the differences were statistically significant in the placebo group. Vitamin D is essential for calcium homeostasis. Vitamin D is metabolized into 25-OH-VITD and then becomes the active form 1, 25-OH-VITD.36 An inadequate serum level of vitamin D affects calcium absorption, resulting in a low serum calcium ion level, which triggers the release of parathyroid hormone, promotes bone absorption, and ultimately leads to osteomalacia or fracture.37 Although we found increased serum levels of calcium and total 25-OH-VITD in the JYMGG group, these increases were not statistically significant; this may be because JYMGG interfered with the absorption of vitamin D in Caltrate D in the human body, but the specific mechanism of the effect is unclear, possibly due to the complex multi-organ and multi-target mechanism of traditional Chinese medicine. This issue warrants further research in future clinical trials.
The present study had some limitations. First, although JYMGG contains epimedium, estrogen-dependent organs (ie, breasts and uterus) were not assessed as safety observation indicators. Second, the efficacy of JYMGG in the treatment of osteoporosis was not evaluated with fracture as the endpoint. Third, the duration of intervention was too short to detect significant differences in BMD and some bone metabolism indicators between the treatment and placebo groups.
Conclusion
In summary, we conducted a prospective study to investigate the efficacy of a Chinese herbal compound in the treatment of osteopenic low back pain. The results showed that JYMGG significantly relieved the symptoms of low back pain in patients with reduced bone mass. This study provides more clinical evidence for the treatment of lumbago and suggests the effectiveness of JYMGG as a new treatment method for patients with lumbago.
Data Sharing Statement
We are willing to share individual deidentified participant data. We will share Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices). Study Protocol, Statistical Analysis Plan, Informed Consent Form, Clinical Study Report, Analytic Code will be made available. Anyone who wishes to access the data can get the above data by sending an email to this email address: [email protected]. These data are available for: beginning 3 months and ending 5 years following article publication.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.This research proposal was approved by the Ethics Committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine on December 7, 2017.(Ethics Number: 2017LCSY056).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
We thank Kelly Zammit, BVSc, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the Shanghai Shenkang Hospital Development Project (16CR3074B), Longhua Hospital-Minhang TCM Specialty Alliance construction project (2021-2023, LM03 Traditional Chinese Orthopedics &Traumatology), and the The fifth batch of dragon Medicine of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine (KC2022006).
Disclosure
Zihao Qin, Ke Xu, Jinhai Xu, Jie Ye, and Wen Mo declare that they have no conflict of interest in this work.
References
1. Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(Suppl 2):S3–S11.
2. Yu F, Xia W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch Osteoporos. 2019;14(1):32.
3. Chou YC, Shih CC, Lin JG, Chen TL, Liao CC. Low back pain associated with sociodemographic factors, lifestyle and osteoporosis: a population-based study. J Rehabil Med. 2013;45(1):76–80.
4. Miyagi M, Inoue G, Murata K, et al. Factors associated with pain-related disorders and gait disturbance scores from the Japanese orthopedic association back pain evaluation questionnaire and Oswestry Disability Index in patients with osteoporosis. Arch Osteoporos. 2021;17(1):1.
5. Urushihara H, Yoh K, Hamaya E, Taketsuna M, Tanaka K. Responsiveness of the Japanese Osteoporosis Quality of Life questionnaire in women with postmenopausal osteoporosis. Health Qual Life Outcomes. 2014;1(12):178.
6. Suzuki M, Orita S, Miyagi M, et al. Vertebral compression exacerbates osteoporotic pain in an ovariectomy-induced osteoporosis rat model. Spine. 2013;38(24):2085–2091.
7. Fujimoto K, Inage K, Orita S, et al. The nature of osteoporotic low back pain without acute vertebral fracture: a prospective multicenter study on the analgesic effect of monthly minodronic acid hydrate. J Orthop Sci. 2017;22(4):613–617.
8. Vellucci R, Terenzi R, Kanis JA, et al. Understanding osteoporotic pain and its pharmacological treatment: supplementary presentation. Osteoporos Int. 2018;29(9):2153–2154.
9. den Uyl D, Geusens PP, van Berkum FN, Houben HH, Jebbink MC, Lems WF. Patient preference and acceptability of calcium plus vitamin D3 supplementation: a randomised, open, cross-over trial. Clin Rheumatol. 2010;29(5):465–472.
10. Camacho PM, Petak SM, Binkley N, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS-2020 UPDATE. Endocr Pract. 2020;26(Suppl 1):1–46.
11. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170.
12. Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. Biomed Res Int. 2018;2018:4840531.
13. Zhang ND, Han T, Huang BK, et al. Traditional Chinese medicine formulas for the treatment of osteoporosis: implication for antiosteoporotic drug discovery. J Ethnopharmacol. 2016;189:61–80.
14. Shi Q. Clinical study on the prevention and treatment of method of consolidating the kidney and supplementing essence. Shanghai J Tradit Chin Med. 1996;10:2–5. Chinese.
15. Cui ZH, Yang Z, Zhao CW, Yin HB, Zhao WH, Shi Q. Effects of different doses of Jianyao Migu Tablets on bone formation and the expression of BMP-7 and OC in mouse bone marrow mesenchymal stem cells. Chin J Tradit Chin Med Pharm. 2017;32(6):2754–2757. Chinese.
16. Tang DZ, Yang Z, Da WW, et al. The effect of different dose JianYao-MiGu tablet on mice bone formation and BMP 7 expression of bone marrow mesenchymal stem cells. Shanghai J Tradit Chin Med. 2016;50(7):
17. Zhu HM, Qin L, Garnero P, et al. The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis. Osteoporos Int. 2012;23(4):1317–1327.
18. Genant HK, Engelke K, Bolognese MA, et al. Effects of Romosozumab Compared With Teriparatide on Bone Density and Mass at the Spine and Hip in Postmenopausal Women With Low Bone Mass. J Bone Miner Res. 2017;32(1):181–187.
19. Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338(11):736–746.
20. Wang YN, Hou QW, Cai YM, et al. Analysis of Bone Mineral Density in Different Parts of Postmenopausal Women in Beicai District of Shanghai. Chin J Primary Health Care. 2021;35(11):59–61.
21. Wang Z, Wang D, Yang D, Zhen W, Zhang J, Peng S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int. 2018;29(3):535–544.
22. Udagawa N, Koide M, Nakamura M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39(1):19–26.
23. Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396.
24. Deeks ED. Denosumab: a Review in Postmenopausal Osteoporosis. Drugs Aging. 2018;35(2):163–173.
25. Ohtori S, Akazawa T, Murata Y, et al. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17(2):209–213.
26. Iwamoto J, Makita K, Sato Y, Takeda T, Matsumoto H. Alendronate is more effective than elcatonin in improving pain and quality of life in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22(10):2735–2742.
27. Abdelaziz DM, Abdullah S, Magnussen C, et al. Behavioral signs of pain and functional impairment in a mouse model of osteogenesis imperfecta. Bone. 2015;81:400–406.
28. Mediati RD, Vellucci R, Dodaro L. Pathogenesis and clinical aspects of pain in patients with osteoporosis. Clin Cases Miner Bone Metab. 2014;11(3):169–172.
29. Li J, Luo M, Wang S, et al. Icariin Ameliorates Lower Back Pain in Rats via Suppressing the Secretion of Cytokine-Induced Neutrophil Chemoatractant-1. Biomed Res Int. 2020;2020:4670604.
30. Kim SM, Ryu V, Miyashita S, et al. Thyrotropin, Hyperthyroidism, and Bone Mass. J Clin Endocrinol Metab. 2021;106(12):e4809–e4821.
31. Sun L, Davies TF, Blair HC, Abe E, Zaidi M. TSH and bone loss. Ann N Y Acad Sci. 2006;2(1068):309–318.
32. Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162.
33. Deng T, Zhang W, Zhang Y, et al. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr Disord. 2021;21(1):49.
34. Ma R, Morshed S, Latif R, Zaidi M, Davies TF. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid. 2011;21(8):897–906.
35. Agrawal M, Zhu G, Sun L, Zaidi M, Iqbal J. The role of FSH and TSH in bone loss and its clinical relevance. Curr Osteoporos Rep. 2010;8(4):205–211.
36. Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–1250.
37. Nakamichi Y, Udagawa N, Suda T, Takahashi N. Mechanisms involved in bone resorption regulated by vitamin D. J Steroid Biochem Mol Biol. 2018;2(177):70–76.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.