Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
A modification of intraluminal middle cerebral artery occlusion/reperfusion model for ischemic stroke with laser Doppler flowmetry guidance in mice
Authors Cai Q, Xu G, Liu J, Wang L, Deng G, Liu J, Chen Z
Received 30 July 2016
Accepted for publication 7 September 2016
Published 3 November 2016 Volume 2016:12 Pages 2851—2858
DOI https://doi.org/10.2147/NDT.S118531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Wai Kwong Tang
Qiang Cai,1,* Gang Xu,2,* Junhui Liu,1 Long Wang,1 Gang Deng,1 Jun Liu,3 Zhibiao Chen1
1Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, 2Department of Neurosurgery, Xiantao First People’s Hospital, Xiantao, 3Department of Emergency, The Central Hospital of Wuhan, Wuhan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Abstract: Stroke is one of the common causes of death and disability in the world. The intraluminal middle cerebral artery occlusion/reperfusion (MCAO/R) model is a “gold standard” in surgical ischemic stroke models. Here, we optimized the procedure of this model by ligating on external carotid artery (ECA) stump and two ligatures prepared on internal carotid artery, which could improve the success and survival rate in mice. The results show that ECA approach was superior to common carotid artery approach. Meanwhile, we found that the exposure of pterygopalatine artery was not an essential step for MCAO/R model in mice.
Keywords: middle cerebral artery occlusion, ischemia stroke, animal model, mice
Introduction
Stroke accounts for ~11.1% of all deaths worldwide and remains a worldwide health burden.1 With no widely effective therapy available, stroke is a cause of serious long-term disability, and the annual expenditure was estimated at $36.5 billion in the US.2 Approximately 87% of stroke2 cases were ischemic strokes and ~70% of infarcts were most commonly caused due to blockage of the middle cerebral artery (MCA) and its branches in humans.3
Rodent models of cerebral ischemia have been important for increasing our understanding of stroke pathophysiology and investigation of potential therapeutics. Over the last three decades, a variety of animal stroke models, including intraluminal MCA occlusion/reperfusion (MCAO/R) model, craniotomy model, photothrombosis model, endothelin-1 model, and embolic stroke model, have been developed. Since the MCAO/R model had the advantage of mimicking human ischemic stroke, exhibiting a penumbra, being highly reproducible, reperfusion being highly controllable and no craniotomy, it became the “gold standard” in surgical ischemic stroke models.4
The MCAO/R model was typically used in rats. Due to the genetically modified (transgenic or knockout) mouse strains providing a unique opportunity to understand the pathophysiological mechanisms of ischemic stroke, this model was introduced into mouse by Chan et al,5 and then it attracted increasing attention.6,7 Although many efforts had been made to improve this model,8,9 the survival rate and success rate remained unsatisfying and diverse among different studies, which may be due to the small size of the blood vessels and the difficulty of the operation procedure. To overcome these shortcomings, we optimized the operative procedure and shortened the surgical time to improve the survival rate of the mice.
Materials and methods
Animals
A total of 60 C57Bl/6J male mice at 10 weeks, weighing 20–25 g, were used in this model. Animals were divided into three groups, with 20 mice in each group. In group A, the pterygopalatine artery (PPA) was identified and temporarily tied, one ligature was prepared on external carotid artery (ECA) stump, and two were ligated on internal carotid artery (ICA). In group B, the PPA was not exposed, two ligatures were prepared on ECA stump, and one ligature was prepared on ICA. In group C, the PPA was not exposed, one ligature was prepared on ECA stump, and two ligatures were prepared on ICA. Mice were subjected to transient MCAO of 60 minutes followed by reperfusion.
All mouse protocols procedures were approved by the Animal Care and Use Committee of Wuhan University and followed the Animal Care and Use guidelines of Wuhan University.
Surgical procedure
Anesthetic depth assessment
Mice were anesthetized with 5.0% isoflurane and maintained on 1.5% isoflurane in 70% N2O and 30% O2 using a small-animal anesthesia system (VetEquip, Pleasanton, CA, USA). Before starting surgery, the depth of anesthesia was assessed by toe pinch. If no response was elicited, the surgery would be started.
Microtip set and measurement of cerebral blood flow (CBF)
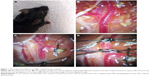
CBF was monitored by using a laser Doppler flowmeter (LDF; PeriFlux System 5000; Perimed, Stockholm, Sweden). An incision (1 cm) was made on skin overlying the calvarium, and the skin was pulled laterally to affix a microtip (MTB 500-OL120). The microtip was placed perpendicular to the surface of the right parietal skull (2 mm posterior and 6 mm lateral to the bregma) to monitor blood flow in the MCA territory and record by laser Doppler Perfusion Monitoring Unit (PeriFlux System 5000 with PF 5010) (Figure 1A).
Transcranial LDF was used to measure CBF in the MCA territory, and only mice with ≥75% flow reduction during the ischemic period were included in this study in order to exclude incomplete ischemia.10
MCAO
The surgical steps of the MCAO were performed as we described previously.11 Briefly, after microtip was attached to skull, the animals were placed on the operating table in supine position under a stereo dissecting microscope (Leica A60). The surgical region was disinfected and covered with a sterile drape. After dissection of the carotid triangle, the common carotid artery (CCA), ECA, ICA, occipital artery (OA), and superior thyroid artery (STA) were identified (Figure 1B). The CCA was temporarily ligated, the ECA was permanently ligated, and the OA and STA were coagulated and cut off (Figure 1C). Two temporary ligatures were prepared around ICA and gently tightened to prevent retrograde blood flow through ICA (Figure 1D). After performing arteriotomy on ECA stump, a nylon monofilament suture (cat no 602156PK5Re; Doccol Corporation, Sharon, MA, USA) was inserted from ECA to ICA. The ligations on ICA were loosened and tightened one by one when the monofilament passed through ICA until a mild resistance was felt. Animals were allowed to awaken from anesthesia during MCAO and returned to their cages. After 60 minutes of occlusion, reperfusion was obtained by withdrawal of the suture and the incision on ECA was electrocoagulation. The surgical procedures had subtle differences among the three groups, and the PPA needed to be dissected and temporarily tied in group A.
Postoperation care
An antibiotic ointment was applied locally, and then the incision was closed. The animals received 0.5 mL of warm saline subcutaneously for volume resuscitation. Appropriate analgesia (ie, meloxicam at 0.3 mg/kg) was used before the surgery and 24 hours after the surgery. During the surgical procedures and 2 hours after reperfusion, the animal body temperature was maintained at 36.5°C±0.5°C using a temperature-controlled heating plate and heat recovery box.
Behavioral assessment
An expanded six-point scale was modified and used for the present investigation. Behavioral assessments were made at 4 hours to 7 days after reperfusion by an individual blinded to the treatment of the mice. The neurological deficits were scored as follows: 0 (no deficit), 1 (failure to extend left forepaw fully), 2 (circling to the left), 3 (falling to the left), 4 (no spontaneous walking with a depressed level of consciousness), and 5 (dead).12
Staining and quantitative measurement of brain infarct volume
After 7 days of MCA reperfusion, the surviving mice were weighed and deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). Mice brains were transcardially perfused with 10% phosphate-buffered saline and then carefully extracted. After chilling for 15 minutes at −20°C, the brains were placed in a brain matrix slicer. Coronal sections were prepared and subjected to 2% triphenyltetrazolium chloride (TTC) staining at 37°C for 20 minutes. Serial sections were photographed using a digital camera, and the areas of infarct were measured using the imageJ program.
Statistical analysis
Data were collected from experiments and presented as mean ± SD. One-way analysis of variance and Mann–Whitney test were used for statistical analysis. All analyses were performed using the SPSS software. Histograms and diagrams were constructed with Graphpad Prism (Version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). The values of P<0.05 were considered statistically significant.
Results
The operation time and mortality rate
All animals tolerated the surgery, and no mice died during the surgery. The average operation time decreased gradually in groups A, B and C, which was 16 minutes, 13 minutes and 12 minutes, respectively. After 7 days of the MCAO/R model, 5 mice, 2 mice, and 1 mouse died in groups A, B and C, and the survival rate was 75%, 90%, and 95% respectively (Table 1). The operation time and the survival rate were significantly different between group A and group B and group A and group C (P<0.05), but there was no significant difference between group B and group C (P>0.05; Figure 2A and B).
  | Table 1 The operation time and survival rate of MCAO/R model in mice |
CBF reduction
Laser Doppler Perfusion Monitoring Unit record showed a significant decrease of 81.8%±4.6%, 84.3%±7.3%, and 85.8%±6.9% in MCA flow from baseline to occlusion in all three groups (P<0.01) and an increase of 88.5%±5.7%, 86.3%±4.5%, and 90.1%±7.2% from occlusion to 5 minutes reperfusion when compared to baseline, respectively (Figure 3), but there was no significant difference among the three groups (P>0.05).
Neurological deficit score
All behavioral tests were carried out at 4 hours and 1–7 days after MCAO/R (Table 2). Neurological evaluations showed that the neurological scores were significantly different between group A and group B and group A and group C (P<0.05), but there was no significant difference between group B and group C (P>0.05; Table 2 and Figures 4 and 5).
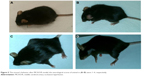  | Figure 5 The mouse’s behavior after MCAO/R model; the neurological scores of animal in (A–D) were 1–4, respectively. |
Staining of brain infarct volume
After TTC staining, the infracted area was shown as a white (unstained) tissue adjacent to red brick (stained) tissue (Figure 6). The infarct volume was calculated by summing the representative areas in all sections and multiplied by the slice thickness. The mean infarct volumes of the three groups were 83.5±8.3 mm3, 89.3±7.5 mm3, and 85.7±6.1 mm3, respectively, with no significant difference among the three groups (P>0.05).
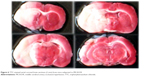  | Figure 6 TTC-stained serial coronal brain sections (2 mm) from mice subjected to MCAO/R. |
Discussion
The intraluminal MCAO/R model mimics one of the most common types of ischemic stroke in humans and was first introduced by Koizumi and later modified by Longa.13 In Koizumi method, a silicon-tipped monofilament was passed through an incision in the CCA, then advanced through the ICA and up to the circle of Willis, and halted at the entrance of the MCA. In the Longa method, a flame-blunted monofilament was used and entered through an incision in the ECA, then guided up to ICA, and reached to MCA. These two methods have been cited directly 205 and 8,472 times, respectively, during the last 30 years,4 and numerous further modifications of this method have been reported in the literature.13
However, the result and outcome were not stable, and a relatively low success rate and high mortality rate were still observed often especially in mice models. A review of literature showed that the infarct variation coefficient of commonly performed stroke models ranged from 5% to 200%.10 Many factors play significant role in causing outcome variation, such as strain-related differences, size of suture tip, duration of occlusion, body temperature, anesthesia, and other factors. For the intraluminal MCAO/R model in mice, the key factors affecting the outcome consistency are the physical properties of the monofilament and the surgical procedure.
Selection of occludes
The physical characteristics of the occlude influence outcome variation by causing insufficient occlusion, premature reperfusion, and/or filament dislodgement. Many occludes are applied in the MCAO/R model, including silicon rubber-coated monofilament, flame-blunted monofilament, poly-L-lysine (PLL)-coated monofilament, methyl methacrylate glue-coated monofilament, silicon resin-coated monofilament, and nail polish-coated monofilament.10 It turns out that silicone rubber-coated monofilaments are superior to flame-blunted monofilaments for producing consistent ischemic brain injuries and less occurrence of subarachnoid hemorrhage (SAH). In the mouse intraluminal model, SAH rate could reach 40% if uncoated heat-blunted monofilaments were being used and such a modeling method resulted in the deviation for infarction volume being >50% of its corresponding mean value.14 The PLL-coated monofilament seemed to only increase the mortality (to a rate of 60%), without any benefits for reducing infarct variation.15
Doccol MCAO sutures have a sufficient length of coated surface, which is cylindrical in shape, elastic, and smooth. Varying sized occludes can be conveniently obtained commercially (www.doccol.com) with desired tip diameter and silicon rubber coating length. The use of Doccol MCAO sutures has been reported to be able to achieve a much better result. For example, the SD for infarction volume was ~5–10% of its corresponding mean value for the 60-minute transient MCAO; the success rate was found to be 96%, and the SAH rate was found to be 0%.10,16,17
MCAO surgical procedure
To produce results relevant to a stroke study, high-quality rodent models are very important in experimental stroke research. There are several elements, surgical skills, and technical details to improve the experimental outcome.
The selection of surgical approach
There are two main surgical approaches for MCAO/R in mice. In Koizumi’s method, the occlude introduced into the ICA via a cut in the CCA which being called CCA approach, so the CCA on the side of the surgery must be permanently tied to prevent bleeding. This approach is a simpler surgical procedure, but at the same time, it changes the dynamics of CBF when the occlude is withdrawn for reperfusion because blood flow will enter only from the contralateral side through the circle of Willis. While in Longa’s method, the occlude introduced into ICA via a cut in ECA which being called ECA approach; the permanently tied artery is ECA, and the CCA is reserved. The ECA approach is a better choice for transient MCAO because it maintains the anatomic integrity required for reperfusion. The results demonstrate that following the Koizumi method, reperfusion reached only 50% of baseline (pre-MCAO) levels, whereas following the Longa method, reperfusion rapidly reached close to 100% of baseline levels,4 and the survival rate is improved twofold using the Longa method when compared with the Koizumi method (26% vs 44% in mortality).4
Shortening the operation time
Operation time is also another influencing factor to the survival and success rate.7 The PPA is a very important artery branch of ICA; some authors suggested to dissect and ligate this artery to guarantee the insertion of the monofilament fiber directly into the MCA,13 but we found that this artery was located at the top of the ICA, and it took much time to dissect it, and it was even more difficult to ligate or clip this artery. In group A, this step took >3 minutes, which maybe contributed to the mortality rate. In groups B and C, we just exposed ICA and adjusted the direction of the filament to the midline, and then, the occludes could reach the terminal ICA successfully. Ansari et al8 also suggested to set up surgical platform to keep the mouse head and neck in a straight direction to avoid deviating the tip of suture into the PPA instead of the ICA. So anatomizing PPA is not an essential step for MCAO/R model, and without doing this procedure we can save time and improve survival rate.
The length of ECA stump is much shorter than the ICA in neck, so it is very hard to make two collar sutures on ECA stump instead of ICA. Although there is no significant difference between these two groups (Groups B and C) in our result, we still believe that two ligations on ICA is much easier, more time-saving, and more suitable for MCAO/R model in mice. Besides, all the string being used making ligations need to wet out to reduce sharp damage on blood vessels.
Prevention of infection
Infection and sepsis are other points that affect the survival rate. Some asepsis procedure needs to be applied to reduce the bacterial infection rate during and after operation. These include preparation of the surgeon, sterile instruments, and separation of surgical and animal prep areas. Before the operation, the surgeon needs to scrub his hands and wear a clean gown, cap, mask, and surgical gloves during surgery. All instruments must be sterilized prior to each group of surgeries and be kept on sterile nonporous drapes during operation.
Laser Doppler flowmetry monitoring
Laser Doppler flowmetry is a method of real-time monitoring of blood flow in MCAO/R model.18 It significantly increases the accuracy of suture positioning and thus ensures obtaining consistent results. This is a noted advantage over less precise techniques of measuring the length of the suture to be inserted, which is susceptible to the interference of anatomical variations.8
Maintain the body temperature
Body temperature is one of the essential factors affecting the extent of infarction, hypothermia decreases the infarct lesion size, and hyperthermia increases the infarct lesion size.7,9 To decrease the variability of the infarction, the mouse body temperature must be maintained steady during the surgery and hypothermia must be avoided after surgery.
Acknowledgments
This study was supported by the Nature Science Foundation of Hubei Province of China (2015CFB182), Hubei Province Health and Family Planning Scientific Research Project (WJ2015MB092), National Natural Science Foundation of China (81671306), Wuhan Science and Technology Plan Project (2016060101010067), and the foundation of China Scholarship Council.
Disclosure
The authors report no conflicts of interest in this work.
References
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. | ||
Go AS, Mozaffarian D, Roger VL, et al. American heart association statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. | ||
Bogousslavsky J, Van Melle G, Regli F. The Lausanne stroke registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19(9):1083–1092. | ||
Smith HK, Russell JM, Granger DN, Gavins FN. Critical differences between two classical surgical approaches for middle cerebral artery occlusion-induced stroke in mice. J Neurosci Methods. 2015;249:99–105. | ||
Chan PH, Kamii H, Yang G, et al. Brain infarction is not reduced in SOD-1 transgenic mice after a permanent focal cerebral ischemia. Neuroreport. 1993;5(3):293–296. | ||
Blanpain C. Tracing the cellular origin of cancer. Nat Cell Biol. 2013;15(2):126–134. | ||
Lee S, Hong Y, Park S, Lee SR, Chang KT, Hong Y. Comparison of surgical methods of transient middle cerebral artery occlusion between rats and mice. J Vet Med Sci. 2014;76(12):1555–1561. | ||
Ansari S, Azari H, McConnell DJ, Afzal A, Mocco J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. J Vis Exp. 2011; (51):2879. | ||
Wu L, Xu L, Xu X, et al. Keep warm and get success: the role of postischemic temperature in the mouse middle cerebral artery occlusion model. Brain Res Bull. 2014;101:12–17. | ||
Liu S, Zhen G, Meloni BP, Campbell K, Winn HR. Rodent stroke model guidelines for preclinical stroke trials (1st edition). J Exp Stroke Transl Med. 2009;2(2):2–27. | ||
Cai Q, Chen Z, Kong DK, et al. Novel microcatheter-based intracarotid delivery approach for MCAO/R mice. Neurosci Lett. 2015;597:127–131. | ||
Tatlisumak T, Carano RA, Takano K, Opgenorth TJ, Sotak CH, Fisher M. A novel endothelin antagonist, A-127722, attenuates ischemic lesion size in rats with temporary middle cerebral artery occlusion: a diffusion and perfusion MRI study. Stroke. 1998;29(4):850–857; discussion 857–858. | ||
Guzel A, Rolz R, Nikkhah G, Kahlert UD, Maciaczyk J. A microsurgical procedure for middle cerebral artery occlusion by intraluminal monofilament insertion technique in the rat: a special emphasis on the methodology. Exp Transl Stroke Med. 2014;6:6. | ||
Tsuchiya D, Hong S, Kayama T, Panter SS, Weinstein PR. Effect of suture size and carotid clip application upon blood flow and infarct volume after permanent and temporary middle cerebral artery occlusion in mice. Brain Res. 2003;970(1–2):131–139. | ||
Huang J, Kim LJ, Poisik A, Pinsky DJ, Connolly ES Jr. Does poly-L-lysine coating of the middle cerebral artery occlusion suture improve infarct consistency in a murine model? J Stroke Cerebrovasc Dis. 1998;7(5):296–301. | ||
Chen Y, Ito A, Takai K, Saito N. Blocking pterygopalatine arterial blood flow decreases infarct volume variability in a mouse model of intraluminal suture middle cerebral artery occlusion. J Neurosci Methods. 2008;174(1):18–24. | ||
Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115(17):2323–2330. | ||
Liu F, McCullough LD. The middle cerebral artery occlusion model of transient focal cerebral ischemia. Methods Mol Biol. 2014;1135:81–93. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.