Back to Journals » Infection and Drug Resistance » Volume 14
A Longitudinal Evaluation of the Bacterial Pathogens Colonizing Chronic Non-Healing Wound Sites at a United States Military Treatment Facility in the Pacific Region
Authors Nahid MA, Griffin JM , Lustik MB, Hayes JJ, Fong KSK, Horseman TS, Menguito M, Snesrud EC, Barnhill JC, Washington MA
Received 20 June 2020
Accepted for publication 23 September 2020
Published 6 January 2021 Volume 2021:14 Pages 1—10
DOI https://doi.org/10.2147/IDR.S260708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Md A Nahid,1 Jaclyn M Griffin,2 Michael B Lustik,3 Jordan J Hayes,3 Keith SK Fong,3 Timothy S Horseman,3 Massimo Menguito,4 Erik C Snesrud5 ,† Jason C Barnhill,4,6 Michael A Washington4
1Department of Dental and Craniomaxillofacial Trauma Research, United States Army Institute of Surgical Research, JBSA Fort Sam, Houston, TX 78234, USA; 2Department of Vascular Surgery, Tripler Army Medical Center, Honolulu, HI, 96859, USA; 3Department of Clinical Investigation, Tripler Army Medical Center, Honolulu, HI 96859, USA; 4Department of Chemistry and Life Science, United States Military Academy, West Point, NY 10996, USA; 5Multidrug-Resistant Organism Repository and Surveillance Network (MRSN), Walter Reed Army Institute of Research, Silver Spring, MD 20910, USA; 6Department of Radiology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA
†Dr Erik C Snesrud passed away on February 10, 2020
Correspondence: Md A Nahid
United States Army Institute of Surgical Research, JBSA Fort Sam, Houston, TX 78234, USA
Tel +1 210-539-6419
Fax +1 210-539-3877
Email [email protected]
Purpose: The biology of chronic wounds is complex and many factors act concurrently to impede healing progress. In this study, the dynamics of microflora changes and their antibiotic susceptibility patterns were evaluated longitudinally over 30 days using data from 28 patients with a total of 47 chronic lower extremity wounds.
Materials and Methods: In this study, colonized wound isolates were characterized using cultural, biochemical, and VITEK 2 methods. Antibiotic susceptibility patterns of the wound isolates were analyzed using various phenotypic assays. Furthermore, antimicrobial resistance patterns and the presence of mutations were evaluated by a genotypic assay, whole-genome sequencing (WGS).
Results: Staphylococcus aureus and Pseudomonas aeruginosa were found to be the most common strains at early time points, while members of Enterobacteriaceae were prevalent at later stages of infection. Antimicrobial resistance testing and whole-genome sequencing revealed that the molecular and phenotypic characteristics of the identified wound pathogens remained relatively stable throughout the study period. It was also noted that Enterobacter and Klebsiella species may serve as reservoirs for quinolone resistance in the Pacific region.
Conclusion: Our observations showed that wounds were colonized with diverse bacteria and interestingly their numbers and/or types were changed over the course of infection. The rapid genetic changes that accompanied the first 4 weeks after presentation did not directly contribute to the development of antibiotic resistance. In addition, standard wound care procedures did not appear to select for resistant bacterial strains. Future efforts should focus on defining those genetic changes associated with the wound colonizing microorganisms that occur beyond 4 weeks.
Keywords: anti-microbial resistance, antimicrobial susceptibility profiling, non-healing wound, wound microflora, wound healing
Introduction
Chronic, non-healing wounds are a significant burden on the affected patient and healthcare systems due to the increased costs associated with wound care. Chronic wounds affect over 6.5 million people annually in the United States, with annual costs greater than $26.8 billion.1 Wound infection may occur in both simple and complex wound environments that affect the wound’s inability to progress through the repair cycle in a timely manner. While low levels of infection and inflammation can be beneficial to wound-healing progress, the colonization of large numbers of bacterial pathogens negatively impacts the wound-healing process.2,3 Wound chronicity is characterized by prolonged (usually 4 weeks to 3 months) inflammation, defective re-epithelialization, and impaired connective tissue remodeling.4 Wound healing is impaired due to multiple factors, including the presence of underlying co-morbidities such as diabetes, neuropathy, immunosuppression, vascular or venous insufficiencies, or the presence of any disease or condition leading to the development of a weakened immune system.5
Although various strategies are currently used to treat non-healing wounds, there is no one standardized therapy. Treatment is determined on a case by case basis and depends on several factors, including co-morbidities and the presence or absence of critical colonizing microorganisms.6 It is unclear whether the microbial profile change has an impact on wound healing. However, it has been established that a detectable transition of the microbial populations colonizing non-healing wounds tends to occur and that this transition follows a predictable pattern. The early acute wound tends to be dominated by Gram-positive bacteria, the majority of which are derived from normal skin flora and typically include S. aureus and various beta-hemolytic Streptococci.7 The most common Gram-negative rods observed in wound infections are Proteus species, Escherichia coli, and Klebsiella species. If surface bacterial colonization persists for an extended period of time and venous insufficiency develops, deep underlying tissues can become colonized with various anaerobic organisms. This is most likely due to the presence of reduced oxygen tension in the deeper tissue that favors the growth of both facultative and obligate anaerobes.8 The most common anaerobic bacteria found in chronic wounds include anaerobic Actinomyces species, Bacteroides species, and Clostridium species.7 Beyond anaerobes, several aerobic Gram-negative rods, including Pseudomonas, Acinetobacter, and Stenotrophomonas have also been found in late-stage chronic wounds.9 Deep tissue wound infections such as pressure ulcers are typically polymicrobial and are often populated by greater than four different bacterial species.9 Despite the fact of numerous studies have evaluated the bacterial populations present in chronic wound sites, there is a paucity of information in the literature regarding the changes that occur in populations of wound flora over time. Along this line, there has never been a comprehensive study on the changes in wound microflora that occur in chronic, non-healing wounds in the Pacific region.
Although chronic-infected wounds have been extensively studied, there are still many questions that have not been adequately addressed. It has been demonstrated that many bacteria can evolve rapidly to utilize new substrates for energy, tolerant degradation of the host immune response, and resist destruction by antibacterial compounds.10 It has also been found that many of the genetic changes necessary for this type of evolution can develop in a bacterial population in less than 500 generations (approximately 30 days) in the laboratory environment.11 However, it is still unclear whether genetic change can occur at the same rate in the wound environment or whether short-term evolution can lead to the conversion of antibacterial susceptible bacteria to drug-resistant phenotypes.
The present study was designed to evaluate the temporal changes in colonizing bacterial populations and antibiotic susceptibility patterns in wound pathogens encountered at Tripler Army Medical Center (TAMC) in Honolulu, Hawaii. Since TAMC routinely treats active duty military members, military retirees, military dependents, veterans, and civilian personnel representing various Asian and Pacific island populations, it is possible that these data are broadly representative of the region as a whole. Ultimately, these data may serve to inform the development and implementation of future diagnostic and treatment strategies for non-healing chronic wounds.
Materials and Methods
Ethics Statement
The institutional review board of the United States Army Human Use Committee at the Regional Health Command-Pacific (RHC-P) approved this study protocol (IRB# 218022). The protocol complied with all ethical considerations involving human subjects and all information obtained following standard clinical guidelines. Investigators adhered to the policies for the protection of human subjects as prescribed in 45 Code of Federal Regulation part 46.12 All of the study participants provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Sample Collection
In this study, 37 wound patients were enrolled between December 2016 and December 2017 at TAMC Vascular Limb Salvage Clinic. Subjects were evaluated and assessed at 3 time points, these were the initial clinic presentation prior to antimicrobial therapy (day 0), 15 ± 2 days post-presentation and the initiation of therapy (day 15), and 30 ± 2 days after initial therapy (Day 30). Of note, after the initial visit (D0), wounds were cleansed with various commercial dressings following standard wound cleansing procedure. The wounds were dressed with silver-based dressings in various forms (gel, foam, contact layer or hydrofiber), Iodine-based gel, collagen, or a debriding agent. The clinician’s selection of the wound care regimen was based on the assessment of the wound. Compression was initiated on venous leg ulcers. In addition to dressing, seven patient wounds were treated with antibiotics based on VITEK 2 antimicrobial sensitivity (bioMérieux, Lyon, France). The combinations were Dakin’s (antiseptic) wash, silver-based dressing and compression with moxifloxacin (400 mg daily for 10 days) and co-amoxiclav (125/500 mg, twice daily for 10 days) or bacitracin only (twice daily) or Iodosorb gel with doxycycline (100 mg twice daily for 10 days). Data were excluded if the patient did not attend the two follow-up visits at days 15 and 30, or if no bacterial growth was found from the specimen collected on the initial visit, leaving a total of 28 patients with 47 wounds for subsequent analysis. No samples were obtained from patients younger than 18 years of age. All samples were devoid of any patient identifiers before analysis. Wounds were sampled by sterile swab per standard of care using aerobic liquid Amies media and anaerobic Vacutainer collection tubes (Becton Dickinson, Franklin Lakes, NJ).
Sample Processing and Identification
The aerobic wound swabs were inoculated onto aerobic media, which included 5.0% Blood agar, MacConkey agar, and Chocolate agar (Becton Dickinson [BD], Sparks, MD). The anaerobic swabs were inoculated in Pre-Reduced, Anaerobically Sterilized (PRAS) anaerobic media (Anaerobe Systems, Morgan Hill, CA), and thioglycollate broth. All inoculated aerobic media were incubated at 37°C for 24–48 hours under aerobic condition and anaerobic media for 2–5 days under anaerobic condition (BD GasPak EZ anaerobe pouch system). All visibly noticeable colonies were isolated for characterization and identification using standard culture (or characteristic morphology) and biochemical testing, including Gram stain, catalase, oxidase, indole, β-lactamase, and Pyrrolidonyl Aminopeptidase (PYR) tests (Hardy Diagnostics, Santa Maria, CA). Additionally, antibiotic (colistin, vancomycin, and kanamycin) sensitivity patterns and aero-tolerance tests were performed for anaerobic characterization. The VITEK 2 automated system (bioMerieux Inc., Durham, NC) was used for further confirmation of the presumptively identified bacterial species and antibiotic susceptibility profiling. All instances of VITEK 2 derived methicillin-resistant S. aureus (MRSA) isolates were further confirmed by a rapid lateral immuno-flow assay procedure (Hardy Diagnostics, Santa Maria, CA). This assay detects altered penicillin-binding protein, PBP2a, which is encoded by the mecA gene.13,14 Instances of VITEK 2 derived extended-spectrum β-lactamases (ESBLs) in the members of the Enterobacteriaceae were confirmed by an ESBL E-test system (bioMerieux Inc., Durham, NC) on Mueller-Hinton agar using 0.5 McFarland standard in 0.85% saline.15,16 All testing was performed following manufacturer instructions. Following VITEK 2 antibiotic profiling, pure bacterial isolates were preserved on Microbank beads (Pro-Lab Diagnostics Inc., Round Rock, TX) for long-term storage at −80°C.
Multi-Drug Resistant Organism Repository and Surveillance Network Analyses
Whole-genome sequencing (WGS) and antimicrobial resistance (AMR or AR) analyses were performed by the Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) located at the Walter Reed Army Institute of Research, Silver Spring, Maryland, USA. Sequencing was performed using the Illumina MiSeq platform (Illumina, San Diego, CA, USA) as previously described.17 It should be noted that all samples were sent to MSRN, except samples from five patients who did not exhibit growth after the initial presentation. Phenotypic analyses for antibiotic susceptibility pattern were performed using three commercial platforms, which included the Microscan Walkaway (Siemens Healthcare, Pennsylvania, USA), VITEK 2 (bioMérieux, Lyon, France), and the Phoenix (BD Diagnostic Systems, Maryland, USA). All antimicrobial susceptibility data were interpreted using the standardized criteria outlined by Clinical Laboratory Standards Institute (CLSI) evaluation data and statistical analysis.18
Results
Study Population
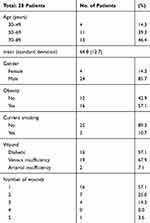
Evaluations by swab and culture were conducted at three time points over a period of 30 days for a total of 28 patients with 47 unique lower extremity wounds. The patients consisted of 24 male patients (85.7%) and 4 female patients (14.3%). Their ages ranged from 37 to 86 years with a median age of 69 (IQR=57-73). Most (75%) had a diagnosis of vascular/arterial insufficiency. Sixteen patients presented with a single wound (57%) and 12 patients had multiple wounds (Table 1). Several of the enrolled patients presented with multiple co-morbidities, including diabetes (57.1%), venous (67.9%) or arterial (7.1%) insufficiency, and smoking (10.7%).
Morphological and Phenotypic Evaluation of the Bacterial Populations Colonizing Chronic and Non-Healing Wounds
Overall Recovery Rate
The rates of isolate recovery tended to vary throughout the course of the study. Colonizing bacterial populations were recovered from each wound site. Overall, 113 bacterial isolates were recovered on day 0, 74 isolates at day 15, and 76 isolates at day 30 from 28 patients. At day 0, at least one bacterial isolate was recovered from all wounds, whereas about 40% of the wounds did not yield any bacteria isolates at days 15 and 30 (Figure 1). The mean number of different species found per wound decreased from 2.4 [standard deviation (std) =1.4] at the initial visit to 1.6 at days 15 (std=1.8) and 30 (std=2.1). It was found that 71% of all positive cultures contained mixed-species communities (79% at day 0, 75% at day 15, and 58% at day 30). Among the isolated organisms, about half were Gram-positive and half were Gram-negative rods (Table 2).
 |
Table 2 Bacteria Species Recovered at Each Visit from 47 Unique Wound Sites from a Total of 28 Patients |
Longitudinal Distribution of Isolate Recovery
At the initial presentation (day 0), the majority of wounds were found to harbor polymicrobial communities (79%). The majority of organisms recovered were aerobic (85%), with a predominance of S. aureus and P. aeruginosa (Table 2). After the initial presentation and initiation of treatment, including topical antiseptic dressing and/or antibiotics, it was found that 19 wounds (40%) lacked bacterial growth on day 15. Of the 28 wounds with growth, cultures from 75% exhibited polymicrobial population (Figure 1). At this time point, the most common organisms among all isolates were aerobic Gram-negative rods (44%) and aerobic Gram-positives (39%). At the 4-week follow-up (day 30), 16 (34%) swabs taken from wounds did not show any bacterial growth. Again, colonized wounds were mostly polymicrobial (58%, 18/31), where S. aureus (26% of all wounds and 16% of all isolates) and P. aeruginosa (21% of all wounds and 13% of all isolates) were still the most prevalent organisms recovered (Figure 1 and Table 2).
There was a general trend of decreasing isolate recovery throughout the course of the study, especially for P. aeruginosa, E. faecalis, E. coli, and P. mirabilis, albeit a small decrease due to overall numbers. While similar trends were not observed for S. aureus and C. koseri that dropped at 2 weeks, however, occurrence went back up at 4 weeks. The occurrence of S. aureus and P. aeruginosa were higher than other isolates. S. aureus was recovered from 18 wounds at day 0, 7 wounds at day 15, and 12 wounds at day 30, while P. aeruginosa was recovered from 16 wounds at day 0, 15 wounds at day 15, and 10 wounds at day 30 (Table 2). It was noted that there was a trend of decreased recovery of both Gram-positive and Gram-negative aerobic organisms throughout the 30-day study period. A significant number of anaerobic organisms, including Fusobacterium species, Prevotella species, Finegoldia species, Peptostreptococcus species, Bacteroides species, and Porphyromonas species were identified (data not shown). These organisms appeared on day 15 and then decreased their frequency over time.
Impact of Treatment on Isolate Recovery
Seven patients (25%) accounting for eleven wounds received antibiotic treatment in addition to standard wound care during the course of the study. Systemic antibiotics such as moxifloxacin, co-amoxiclav, doxycycline, ciprofloxacin, and vancomycin were used to treat these patients in combination with various dressing. Although these patients were treated with antibiotics in accordance with the standard of care, findings have been re-directed to focus on the isolated organisms. Of the wounds surveyed from these patients, no organisms were isolated from five wounds. In the other six wounds, P. aeruginosa was detected from each wound at least from one time point. Interestingly, of the seven patients being treated with antibiotics, S. aureus was isolated from three subjects, two of which were methicillin-resistant. Although no trends were apparent with the antibiotic-treated wounds, other common organisms isolated were Fusobacterium sp., C. koseri, K. pneumoniae, E. coli, Streptococcus agalactiae, and S. epidermidis.
Molecular Evaluation of the Antimicrobial Resistance Determinants of the Bacterial Populations Colonizing Chronic and Non-Healing Wounds
In this study, a total of 223 isolates collected from 23 patients were submitted to MRSN for WGS and AMR testing. Initial phenotypic testing among eight patient wounds revealed 10 distinct MRSA isolates and one ESBL producing Klebsiella species. PBP2a is well defined to render MRSA strains resistant to virtually all β-lactam antibiotics. Of note, lysed MRSA showed a positive reaction for PBP2a, whereas methicillin-sensitive S. aureus was unreactive, as shown by many studies.13,14 Upon ESBL E-test, ESBL Klebsiella species showed a MIC (minimum inhibitory concentration) of ≥8.0 for either CT/CTL (cefotaxime/cefotaxime + clavulanic acid) or TZ/TZL (ceftazidime/ceftazidime + clavulanic acid), similar to other studies.15,16 Testing was performed on each isolate to confirm both genus and species level identification, phenotypic antibiotic susceptibility pattern, and to identify the presence or absence of antimicrobial resistance agents. Numerous mutations were identified from the isolate set at multiple time points; however, none of the mutations were found in a gene that has been shown to impact known antibiotic resistance mechanisms.
Antimicrobial Resistance Genes Detected
The majority of isolates harboring antimicrobial resistance genes were found to harbor genes conferring resistance to the β-lactams (n=63), these were followed by isolates harboring genes conferring resistance to the aminoglycosides (n=46), macrolides (n=27), and fosfomycin (n=24) (Table 3). There were only five isolates that possessed genes conferring resistance to the sulfonamides. Overall, isolates of E. coli, S. aureus, and P. aeruginosa were found to harbor the largest numbers of resistance genes (Table 4). Based on literature searches and meta-information found in AMRFinderPlus and NCBI protein database,19 antimicrobial effectiveness genes were identified and characterized in the wound isolates, as has been outlined in Table 4. Representatives of each of these organisms were found to contain genes capable of conferring resistance to the β-lactams and aminoglycosides. Of note, genes that conferred resistance to the chloramphenicols and tetracyclines were present both in E. coli and S. aureus. Genes conferring resistance to fosfomycin were present in both E. coli and P. aeruginosa. Sulfonamide resistance-conferring genes were only present in E.coli, while genes conferring resistance to streptomycin and tetracycline were present only in isolates of S. aureus (Table 4). Genes capable of conferring resistance to quinolones were only found in Enterobacter cloacae, K. aerogenes, and K. pneumoniae. These isolates were found to contain genes coding for the protein products necessary to produce members of the plasmid-borne OqxAB efflux pump. Surprisingly, none of these isolates were resistant to the quinolones indicating that these genes are inactive and that these isolates may serve as environmental reservoirs.
Longitudinal Resistance Patterns
Overall, the results of the antibiotic resistance testing of each of the isolates that were recovered in this study revealed that between days 0 and 30, no significant changes in antibiotic resistance patterns occur and that most isolates did not convert from a state of antimicrobial sensitivity to resistance or vice versa during the course of infection. One notable exception to this observation was a set of three S. aureus isolates recovered from two distinct wound sites on a single patient. Genomic analysis indicated that these isolates were the same strain. Initially, the isolates had similar antibiotic resistance patterns, a conversion from a sensitive to resistant phenotype for clindamycin was identified in a day 30 isolate, and a conversion from a sensitive to resistant phenotype for erythromycin was detected in a day 15 isolate (Table 5). While a large number of single nucleotide polymorphisms (SNPs) were detected in this particular group of isolates, none of them were associated with genes known to be involved in the development of antibiotic resistance. Rather, comparative genomics showed that the majority were found to be localized to those genes necessary for housekeeping functions and/or maintaining metabolic activities. A similar trend was observed for all isolates evaluated in this study (Table 6).
 |
Table 5 Phenotype Conversion of S. Aureus from Sensitive (S) to Resistant (R) for Clindamycin and Erythromycin Over a Period of 30 Days |
Discussion
Acute or traumatic wounds in normal, healthy individuals usually heal through the regular phases of hemostasis, inflammation, proliferation, and remodeling. While inflammation is a normal part of the wound-healing process, chronic or non-healing wounds stall with sustained inflammation resulting in slow or no indication of improvement weeks after initial presentation.20 The delayed healing time can be attributed to many local and systemic factors, including oxygenation, inflammatory mediators, infection, sex hormones, stress, medications, obesity, alcohol consumption, smoking, and nutrition.21 Additionally, patient age or underlying comorbidities such as diabetes and dryness of wounds can also have a significant effect on wound-healing progress.22,23 As a consequence, the longer the time required for healing, the more diverse microbial population can colonize in the wound and complicate treatment strategies. Thus, proper identification of chronic wounds in terms of continued infection and antimicrobial resistance is necessary to develop an effective treatment plan. The negative impact of bacterial colonization and invasive infection can also modulate wound-healing phases and increase the overall morbidity and mortality associated with chronic wounds.24
An open wound is a favorable place for microbial contamination, colonization, and infection. Once the skin is impaired, normal skin flora, exogenous bacteria, and fungi can gain access to underlying tissues.25 This environment offers a humid, warm, and nutrient-rich niche for the development of synergistic microbial interaction.26 Previous studies have demonstrated that both S. aureus and P. aeruginosa, two of the most common wound pathogens, tend to display higher antibiotic tolerance when cultured together, than separately as is typically done in clinical laboratories.27 The initial bacteria recovered in this study were isolated from wound sites colonized with polymicrobial communities of microorganisms with a majority of P. aeruginosa and S. aureus. It is tempting to speculate that synergistic interactions between these bacteria were responsible for at least some of the pathological features associated with the wound infections.
Anaerobic bacteria constitute a significant proportion of the normal microbiota colonizing skin and various mucosal surfaces of the human body.28 Anaerobes are more commonly found in polymicrobial aerobic and anaerobic infections of endogenous origin. Once anaerobic species are established, obstruction of phagocytosis can occur to prevent the destruction of other microorganisms present. Furthermore, the nutrient flux from one bacterium may sustain the evolution and proliferation of another.29 In this study, a number of anaerobic bacteria were identified. However, the numbers of anaerobe isolations were found to be significantly less than aerobic bacteria, which prompted us to investigate thoroughly the aerobic bacterial population. This observation may be explained by the fact that the wounds were only followed for 30 days and anaerobic organisms tend to proliferate slower than aerobic organisms.30
Seven patients were on antibiotic treatment with a combination of wound dressings, whose cellulitis/wounds improved over the course of infection. Although it would have been interesting to examine wound-healing progression over an extended time, antimicrobial resistance and susceptibility changes were the main focus of this study. With regard to antibiotic therapy in chronic wounds, there is a lack of evidence concerning its effectiveness, optimal regimens or clinical indications for treatment.31 Future studies should focus on long-term evaluation of antibiotic treatment effectiveness as it impacts the wound-healing outcome. Information regarding antibiotics effectiveness during the course of wound infection could help guide and select an optimal wound treatment strategy.
Numerous antimicrobial resistance genes have been detected in this study. The most commonly detected genes were those which are capable of conferring resistance to the β-lactams, the aminoglycosides, the macrolides, and fosfomycin. This finding is significant given that antibiotics from each of these classes are routinely prescribed to treat a wide variety of bacterial infections.31 Surprisingly, conversion from a state of antimicrobial sensitivity to resistance was not detected in the majority of organisms that were evaluated. No single nucleotide polymorphism was found to occur in any of the genes known to alter antibiotic susceptibility. These findings indicate that the bacterial populations that infect chronic or non-healing wounds tend to have relatively stable genomes and that clinically significant genotypic or phenotypic changes did not occur within the first 30 days after presentation to the wound clinic. One set of S. aureus isolates that showed a conversion of antibiotic sensitive to a resistance phenotype, did not belong to the detectable variations in known antimicrobial resistance agents. Due to the study design, we did not extend the study for over 30 days. Our wound clinic only possesses a standard of care culture and sensitivity results. It would be of interest to have an extended antibiogram investigation for the isolates recovered from patients prescribed antibiotics.
Perhaps the most interesting and concerning result of this study was the identification of the plasmid-encoded genes for the production of the multi-drug efflux pump, OqxAB, which was present in all members of the isolated Klebsiella and Enterobacter species over the course of the 30-day evaluation. This system is considered to be one of the most effective mechanisms of plasmid-mediated quinolone resistance.32 The gene oqxAB was first identified on a plasmid that was isolated from isolates of E. coli that were recovered from swine manure in Denmark in 2003.33 This plasmid is highly transmittable in E. coli and among members of the Enterobacteriaceae. Recently, it was suggested that Klebsiella species may serve as an environmental reservoir for these plasmids as well as for the multi-drug efflux pump that they encode.34 If this is true then the detection of the oqxAB gene complex in Klebsiella species isolated from chronic wounds isolated at a military hospital in Hawaii may indicate that this organism may serve as a reservoir for it in the Pacific region and that patients with wound infections caused by members of the Enterobacteriaceae should be monitored for the development of fluoroquinolone resistance.
Conclusion
Wound healing is a very complex and multifactorial process. This is the first study addressing the varying patterns of bacterial wound colonization as well as the genotypic characterization of antibiotic resistance patterns in the mid-Pacific region. Simultaneously, it demonstrates that temporal organism shifts occur similarly in this population compared to other studied geographical locations and patient populations. The results of this study provide evidence that these bacterial populations are relatively stable (genetically and phenotypically) with respect to their species composition and antibiotic resistance profile. Debridement and the use of antimicrobial dressings did not directly select for antimicrobial-resistant bacterial strains. However, the potential for the horizontal spread of antimicrobial resistance determinants has been detected and conversion from a sensitive to a resistant phenotype (although rare) can occur in the early phases of wound care. A prolonged evaluation of horizontal gene transfer mechanisms can be investigated to recognize drug resistance in wound-associated bacteria. When the current data is combined with data collected in future studies, it will have the potential to allow clinicians to develop personally and regionally targeted therapeutic strategies for the management and resolution of chronic or impaired wound infections.
Acknowledgments
This work was supported by the US Department of Defense (DoD) Global Emerging Infections Surveillance (GEIS) program. We acknowledge the staff at the Walter Reed Army Institute of Research-Multidrug-Resistant Organism Repository and Surveillance Network (WRAIR/MRSN) for whole-genome sequencing and bioinformatics analyses, visualization, interpretation, and reporting. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the US Army. This research protocol was approved by the Tripler Army Medical Center Scientific Review Committee. The views expressed in this manuscript are those of the author(s) and do not reflect the official policy or position of the US Military Academy, the Department of the Army, the Department of Defense, or the US Government.
Disclosure
The authors affirm that they have no competing interests in this work.
References
1. Padula WV, Delarmente BA. The national cost of hospital-acquired pressure injuries in the United States. Int Wound J. 2019;16(3):634–640. doi:10.1111/iwj.13071
2. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–96. doi:10.1097/00001432-200404000-00004
3. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77(3):637–650. doi:10.1016/S0039-6109(05)70572-7
4. Krishnaswamy VR, Mintz D, Sagi I. Matrix metalloproteinases: the sculptors of chronic cutaneous wounds. Biochim Biophys Acta Mol Cell Res. 2017;1864(11):2220–2227. doi:10.1016/j.bbamcr.2017.08.003
5. Ackermann PW, Hart DA. Influence of Comorbidities: neuropathy, Vasculopathy, and Diabetes on Healing Response Quality. Adv Wound Care. 2013;2(8):410–421. doi:10.1089/wound.2012.0437
6. Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care. 2015;4(9):560–582. doi:10.1089/wound.2015.0635
7. Tong MJ. Septic complications of war wounds. JAMA. 1972;219(8):1044–1047. doi:10.1001/jama.1972.03190340050011
8. Finegold SM. Host factors predisposing to anaerobic infections. FEMS Immunol Med Microbiol. 1993;6(2–3):159–163. doi:10.1111/j.1574-695X.1993.tb00319.x
9. Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage. 1999;45(8):23–27.
10. Miskinyte M, Sousa A, Ramiro RS, et al. The genetic basis of Escherichia coli pathoadaptation to macrophages. PLoS Pathog. 2013;9(12):e1003802. doi:10.1371/journal.ppat.1003802
11. Bennett AF, Hughes BS. Microbial experimental evolution. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R17–25. doi:10.1152/ajpregu.90562.2008
12. Rice TW. The historical, ethical, and legal background of human-subjects research. Respir Care. 2008;53(10):1325–1329.
13. Hussain Z, Stoakes L, Garrow S, Longo S, Fitzgerald V, Lannigan R. Rapid Detection of mecA-Positive andmecA-Negative Coagulase-Negative Staphylococci by an Anti-Penicillin Binding Protein 2a Slide Latex Agglutination Test. J Clin Microbiol. 2000;38(6):2051–2054. doi:10.1128/JCM.38.6.2051-2054.2000
14. Louie L, Matsumura SO, Choi E, Louie M, Simor AE. Evaluation of three rapid methods for detection of methicillin resistance in Staphylococcus aureus. J Clin Microbiol. 2000;38(6):2170–2173. doi:10.1128/JCM.38.6.2170-2173.2000
15. Farber J, Moder K-A, Layer F, Tammer I, Konig W, Konig KB. Extended-Spectrum Beta-Lactamase Detection with Different Panels for Automated Susceptibility Testing and with a Chromogenic Medium. J Clin Microbiol. 2008;46(11):3721–3727. doi:10.1128/JCM.00777-08
16. Landman D, Bratu S, Kochar S, et al. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae in Brooklyn, NY. J Antimicrob Chemother. 2007;60(1):78–82. doi:10.1093/jac/dkm129
17. Snesrud E, Ong AC, Corey B, et al. Analysis of Serial Isolates of mcr-1-Positive Escherichia coli Reveals a Highly Active ISApl1 Transposon. Antimicrob Agents Chemother. 2017;61(5):5. doi:10.1128/AAC.00056-17
18. Performance standards for antimicrobial susceptibility testing. 23th Informational Supplement. M100-S23 Clinical and Laboratory Standards. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
19. Feldgarden M, Brover V, Haft DH, et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob Agents Chemother. 2019;63(11):11. doi:10.1128/AAC.00483-19
20. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–378. doi:10.1111/bjd.13954
21. Demidova-Rice TN, Durham JT, Herman IM. Wound Healing Angiogenesis: innovations and Challenges in Acute and Chronic Wound Healing. Adv Wound Care. 2012;1(1):17–22. doi:10.1089/wound.2011.0308
22. Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923. doi:10.1002/jps.21210
23. Gould LJ, Fulton AT. Wound Healing in Older Adults. R I Med J. 2016;99(2):34–36.
24. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. Journal of the American Academy of Dermatology. 2016;74(4):607–625. doi:10.1016/j.jaad.2015.08.070
25. Rhody C. Bacterial infections of the skin. Prim Care. 2000;27(2):459–473. doi:10.1016/S0095-4543(05)70207-4
26. Banu A, Noorul Hassan MMN, Rajkumar J, Srinivasa S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas Med J. 2015;8(9):280–285. doi:10.4066/AMJ.2015.2422
27. DeLeon S, Clinton A, Fowler H, Everett J, Horswill AR, Rumbaugh KP. Synergistic Interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an In Vitro Wound Model. Infect Immun. 2014;82(11):4718–4728. doi:10.1128/IAI.02198-14
28. Murphy EC, Frick I-M. Gram-positive anaerobic cocci – commensals and opportunistic pathogens. FEMS Microbiol Rev. 2013;37(4):520–553. doi:10.1111/1574-6976.12005
29. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–269.
30. Hobson PN, Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967;47(1):53–65. doi:10.1099/00221287-47-1-53
31. Tzaneva V, Mladenova I, Todorova G, Petkov D. Antibiotic treatment and resistance in chronic wounds of vascular origin. Clujul Med. 2016;89(3):365–370.
32. Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 2006;6(10):629–640. doi:10.1016/S1473-3099(06)70599-0
33. Rodriguez-Martinez JM, Diaz de Alba P, Briales A, et al. Contribution of OqxAB efflux pumps to quinolone resistance in extended-spectrum- -lactamase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2013;68(1):68–73. doi:10.1093/jac/dks377
34. Hansen LH, Sorensen SJ, Jorgensen HS, Jensen LB. The Prevalence of the OqxAB Multidrug Efflux Pump amongst Olaquindox-Resistant Escherichia coli in Pigs. Microbial Drug Resistance. 2005;11(4):378–382. doi:10.1089/mdr.2005.11.378
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.