Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Aβ Influx into the Blood Evoked by Different Blood Aβ Removal Systems: A Potential Therapy for Alzheimer’s Disease
Authors Kitaguchi N , Kawaguchi K, Sakata M, Aoki H, Yamazaki K, Kaneko M, Kinomura J, Kato M, Hasegawa M , Suzuki N, Mizuno M, Yuzawa Y
Received 28 April 2021
Accepted for publication 1 July 2021
Published 13 July 2021 Volume 2021:17 Pages 2291—2308
DOI https://doi.org/10.2147/NDT.S317104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Nobuya Kitaguchi,1 Kazunori Kawaguchi,1 Miwa Sakata,1 Hiroki Aoki,1 Kazunori Yamazaki,1 Megumi Kaneko,1 Jun Kinomura,1 Masao Kato,2 Midori Hasegawa,2 Nobuo Suzuki,3 Masao Mizuno,4 Yukio Yuzawa2
1Faculty of Clinical Engineering, School of Medical Sciences, Fujita Health University, Toyoake, Aichi, 470-1192, Japan; 2Department of Nephrology, School of Medicine, Fujita Health University, Toyoake, Aichi, Japan; 3Chiryu Clinic, Chiryu, Aichi, Japan; 4Mizuno Clinic, Toyoake, Aichi, Japan
Correspondence: Nobuya Kitaguchi
Faculty of Clinical Engineering, School of Health Sciences, Fujita Health University, 4-8-17, Yuzato, Higashisumiyosi-Ku, Osaka, 546-0013, Japan
Tel +81-6-4392-7087
Fax +81-6-4392-7087
Email [email protected]
Purpose: Amyloid-β (Aβ) is a brain protein that causes Alzheimer’s disease. We have revealed that extracorporeal blood Aβ-removal systems evoked a large Aβ influx into the blood. This study investigated the system that is more effective in evoking Aβ influx.
Methods: Aβ removal activities were compared between hexadecyl-alkylated cellulose beads (HexDC) and fragments of polysulfone hollow fibers (PSf-HFs) in mini-columns to eliminate the filtration effect. Then, adsorptive filtration systems were adapted for PSf hemodialyzers to enhance Aβ adsorption on micropores in the wall of hollow fibers. Plasma Aβ concentrations of patients with renal failure were analyzed during treatment with PSf hemodialyzers alone for 8 h or tandemly connected HexDC and PSf hemodialyzers for 4 h.
Results: In the in vitro study, Aβ removal efficiency for HexDC was approximately 100% during the 60 min treatment, whereas the removal efficiency for PSf-HF fragments gradually decreased. However, PSf hemodialyzer in adsorptive filtration systems removed Aβs comparably or more than HexDC. Aβ influx into the blood increases time-dependently. Concomitant use of HexDC and PSf hemodialyzer evoked a larger Aβ1– 40 influx than that of PSf hemodialyzer alone. However, Aβ1– 42 influx by PSf hemodialyzer alone was similar to or a little larger than influx by the combined system. Both systems evoked almost doubled Aβ influx than estimated Aβs existing in the normal brain during the 4 h treatment.
Conclusion: PSf hemodialyzer alone for a longer period and concomitant use of HexDC and PSf hemodialyzer for a shorter time effectively evoked a larger Aβ influx. To evoke Aβ1– 42 influx, PSf hemodialyzer alone was effective enough. These findings of devices and treatment time may lead to optimal clinical settings for therapy and prevention of Alzheimer’s disease.
Keywords: amyloid, blood purification, extracorporeal system, polysulfone hemodialyzer, HexDC, 3D printer
Introduction
Amyloid-β (Aβ) in the brain is a causative protein of Alzheimer’s disease (AD). Harmful proteins primarily include 40 amino-acid Aβ1–40, 42 amino-acid Aβ1–42, and abnormally phosphorylated tau.1 Impaired Aβ clearance from the brain might increase brain Aβ, particularly in sporadic AD cases. Aβ clearance from the brains of patients with sporadic AD was 30% lower than the clearance in normal subjects, although Aβ production was similar.2 The peripheral administration of anti-Aβ antibodies to increase the clearance of Aβs from the brain has been investigated. Unfortunately, most clinical trials using anti-Aβ antibodies did not meet the endpoint criteria, although brain Aβs were reduced. However, a recent re-analysis of the clinical results for Aducanumab, an anti-Aβ antibody, revealed reduced cognitive decline at the high dose,3 resulting in the accelerated approval of an Alzheimer’s drug by the FDA on June 7, 2021. Furthermore, Gantenerumab, an anti-Aβ antibody used in the dominantly inherited Alzheimer’s network trial unit (DIAN-TU), reduced Aβs and tau in the brain.4,5 Therefore, Aβ may be the primary causative protein for AD.4
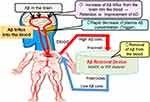
We proposed extracorporeal blood Aβ removal systems (E-BARS) as another method for enhancing Aβ clearance from the brain (Figure 1).6 There are several rationales for blood Aβ removal systems. First, there is an Aβ concentration gradient between the brain and blood. Aβ concentration in the cerebrospinal fluid (CSF) of patients with AD is approximately 100 times higher than in the plasma.7,8 Second, Aβ moves from the brain into the blood via transporters, including low-density lipoprotein receptor-related protein 1 (LRP-1), ApoJ, ApoE, and receptor for advanced glycation end products (RAGE).9–11 Pharmaceuticals targeting RAGE and LRP-1 are being developed to increase Aβ transportation.12
We have reported the following evidence to support the efficacy of blood Aβ removal for AD therapy and prevention. First, we found several medical materials of high efficacy for Aβ removal from the blood, including hexadecyl alkylated cellulose beads (HexDC)6,13 and hollow fibers in hemodialyzers, such as polysulfone (PSf), polymethylmethacrylate, and polyethersulfone.14 The Aβ removal efficiency of PSf dialyzers was as high as 50% for both Aβ1–40 and Aβ1–42 during a 4 h hemodialysis session.15,16 The mechanism of blood Aβ removal by these hemodialyzers is mainly by adsorption.14,17 Second, the removal of blood Aβ evoked a large influx of Aβ into the blood from certain tissues during hemodialysis.15,16,18,19 Third, we showed that Aβ accumulation in the brains of patients undergoing hemodialysis was significantly lower than Aβ accumulation in age-matched controls not undergoing hemodialysis.20 Furthermore, we reported more direct evidence showing that hemodialysis decreased Aβ accumulation in the brain by positron emission tomography (PET)-imaging with Pittsburgh compound B (PiB) as a probe (PiB/PET).21 The brain Aβ accumulation in a chronic renal failure patient suffering from mild cognitive impairment was decreased by −0.19 standard uptake value ratio (SUVR) after hemodialysis for 6 months (4 h/session, three sessions/week). The data suggest that the brain is an origin of Aβ influx into the blood during hemodialysis. Fourth, Aβ concentration in the CSF was decreased in rat studies using HexDC columns. Plasma Aβ concentrations increased during blood Aβ removal, despite the 90% efficiency of Aβ removal using HexDC columns.22 Finally, the mini-mental state examination scores of hemodialysis patients were maintained or slightly improved in a prospective study.18 We also reported that longer hemodialysis duration correlated with lower dementia risk in an analysis of more than 200,000 Japanese hemodialysis patients.23 Furthermore, we recently reported that Aβ oligomers were effectively removed by size-separation methods with hollow fiber devices of appropriately large pore sizes in vitro and in humans.24
Several other groups also reported that removing blood Aβs may be a helpful AD therapy. Peritoneal dialysis, in which peritoneal membranes are used as dialysis membranes instead of hemodialyzers, reduced plasma Aβ in humans and brain Aβ in mouse AD models.25 The authors reported that peritoneal dialysis only removed 131.33 ng of Aβs,25 whereas hemodialysis removed 7219 ng of Aβ1–40 and 664 ng of Aβ1–42, totaling 7883 ng of Aβs as we reported previously.19 Furthermore, plasma exchange therapy, which removes plasma Aβ by discarding the plasma with albumin replacement as Aβ binding protein, effectively improves cognitive functions in patients with AD.26 This therapeutic plasma exchange discarded all plasma proteins, including coagulation factors and immunoglobulins. Therefore, intravenous immunoglobulin was injected every 4 months.
Thus, the removal of blood Aβ has attracted attention as a therapeutic strategy for AD.27
Furthermore, regarding Aβ influx into the blood, we reported that the total amount of Aβ influx during one hemodialysis session (4 h) was calculated as 7219 ng for Aβ1–40 and 664 ng for Aβ1–42 for 30 patients undergoing hemodialysis.19 These amounts of Aβ influx during one hemodialysis session of 4 h are comparable with the reported total Aβ content of 7760 ng in the brain.28
We found several effective materials suitable for blood Aβ removal, including HexDC and PSf-HFs. This study aimed to determine which materials are more efficient at removing Aβs from the blood (Figure 1). Another and primary objective in this study was what systems are more effective to evoke a larger Aβ influx into the blood. We compared two systems: one was a system of enhanced Aβ removal activity by using doubled Aβ removal devices (concomitant use of HexDC and PSf hemodialyzer) for 4 h, the other was a longer treatment system by using PSf hemodialyzer alone for 8 h.
Materials and Methods
Ethical Approval and Collection of Human Plasma
This study conformed to the Declaration of Helsinki’s Good Clinical Practice. This research was comprehensively reviewed and approved by the institutional review board at Fujita Health University (the latest approval number is HM16-266). Patients undergoing blood purification provided written informed consent for collecting blood samples.
Preparation of the Aβ Solutions
Human plasma remaining in used bags of fresh frozen plasma and discarded plasma from patients was obtained after plasma exchange therapy. The plasma was refrozen at −80°C with additional heparin. Before use, the plasma was thawed and shook in a water bath at 37°C and centrifuged to remove precipitates. Detailed procedures were previously described.24
Preparation of Mini-Columns of HexDC Beads and PSf-HF Fragments
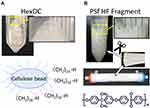
HexDC was chosen for this study because it is one of the most efficient adsorbents for blood Aβ removal, as described in the Introduction.6,13 HexDC beads, shown in Figure 2A, came from a commercially available HexDC device (LIXELLE S-35 containing 350 mL HexDC, Kaneka, Osaka, Japan), which is used to remove β2-microglobulin from the blood of hemodialysis patients having carpal tunnel syndrome in the clinical circuit with tandem connection to hemodialyzers. Hexadecyl alkyl chains are appropriately hydrophobic, interacting with hydrophobic proteins, such as β2-microglobulin and Aβs. HexDC beads (1.75 mL, 1/200 of LIXELLE S-35) were put into a 2.5 mL polypropylene syringe to form the mini-HexDC column.
PSf-HFs were also used in this study as one of the most efficient adsorbents for blood Aβ removal.14–17 Among various materials of hollow fibers in hemodialyzers, PSf is hydrophobic enough to adsorb hydrophobic proteins, such as albumin and Aβs. PSf-HF was obtained from hemodialyzers (APS-13EA, Asahi Kasei Medical, Tokyo, Japan) and cut into 2–5 mm fragments (Figure 2B) to eliminate the filtration effect. The membrane surface area of the fragments in the mini-column of PSf-HF was 0.01 m2, which was 1/200 of 2.0 m2 (a representative membrane area used in clinical hemodialysis). The fragments were put into a 2.5 mL syringe to form a mini-column of PSf-HF fragments.
Aβ Removal with Mini-Columns in vitro
Eighteen milliliters of pooled human plasma was applied continuously to mini-columns of HexDC or PSf-HF fragments and returned to the plasma pool at a flow rate of 240 μL/min using a Perista pump (ATTO, Tokyo, Japan), as shown in Figure 3A. This plasma flow rate was set to 1/200 of the flow rate in a clinical setting with 50 mL plasma/min (77 mL blood/min when the hematocrit is 35%), determined in our previous study.14
The Aβ removal efficiency of the column was calculated as follows: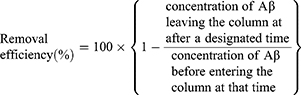
The Aβ reduction rate for the experimental pool solution was defined as follows: 
Aβ Removal by One-Pump Adsorptive Filtration Using Hemodialyzers in vitro
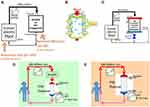
The primary mechanism of Aβ removal using hemodialyzers is adsorption, not filtration.14 To enhance adsorption to the surface of “micropores in the hollow fiber walls” of hemodialyzers (Figure 3B), we developed “adsorptive filtration” systems with two pumps.17 One pump is for blood circulation and the other for filtration. In this study, to make adsorptive filtration systems with two pumps easier to use in clinical settings, one-pump adsorptive filtration systems were created with Venturi tubes instead of filtration pumps (Figure 3C). The Venturi tube has a narrow pass in the middle part, which increases fluid pressure before the narrow pass and decreases the pressure after the narrow pass. The increased fluid (blood) pressure enhances filtration through the wall of hollow fiber membranes in adsorptive filtration systems.
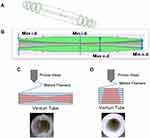
The Venturi tubes were designed with three-dimensional computer-aided design software, DesignSpark Mechanical (RS Components, Yokohama, Japan). Using the designed standard triangulated language (STL) data, Venturi tubes were produced with 1.75 mm filaments of polylactic acid using a fused deposition modeling 3D printer (Replicator 2; MakerBot, NY, USA). In the experimental circuit (Figure 3C), a Venturi tube was added after the polysulfone dialyzer outlet (APS-13EA, Asahi Kasei Medical, Tokyo, Japan) to control the filtration rate. A flowmeter (Coriolis Flow Meter FD-SS02A, Keyence, Osaka, Japan) was placed between the filtrate outlet (one of the dialysate ports) and the junction with the return tube to the pool of 250 mL of human plasma. Human plasma was circulated at a 50 mL/min flow rate using peristaltic pumps (Masterflex Variable-Speed Drive; Cole-Parmer, IL, USA). This flow rate was determined based on our previous studies of adsorptive filtration.14,17
Aβ Removal in Hemodialysis Patients Using PSf Hemodialyzers and HexDC
Table 1 summarizes the demographics of the blood treatment subjects. All subjects were nondiabetic, as diabetes mellitus is an AD risk factor. Three hemodialysis patients having carpal tunnel syndrome underwent treatment using PSf hemodialyzers (APS-21SA, Asahi Kasei Medical, Tokyo, Japan) and tandemly connected HexDC (LIXELLE S-15, Kaneka, Osaka, Japan). Figure 3D shows the clinical circuit for these treatments. Treatment time was 4 h, and patients were treated during the day. Dialysate flowed outside PSf-HF in the PSf hemodialyzer at a flow rate of 500 mL/min.
 |
Table 1 Demographics of the Subjects Undergoing Blood Purification |
This treatment system can be regarded as a kind of E-BARS “with double Aβ-removal devices” (concomitant use of HexDC and PSf hemodialyzer). Furthermore, PSf hemodialyzer in this system worked as an adsorptive filtration system14,17 because of water removal by filtration. Hemodialysis patients can pass little or no urine because of renal failure; excess water by drinking and eating should be removed from the blood during hemodialysis. The filtration rate for the removal of excess water from the blood was approximately 10 mL/min (Figure 3D).
Three additional patients were treated using only PSf hemodialyzers (APS-21SA) for 8 h overnight because they had daytime jobs. Figure 3E shows the clinical circuit. Dialysate flowed outside PSf-HF in the PSf hemodialyzer at a flow rate of 400 mL/min. This treatment system can be regarded as “longer time treatment” of E-BARS. The first 4 h of hemodialysis in these patients was regarded as the hemodialyzer-only (without HexDC) control group compared with concomitant treatments with HexDC and hemodialyzer. Filtration rates of the hemodialyzers were 6–9 mL/min for removal of excess blood water (Figure 3E).
Blood samples were obtained at 0, 1, and 4 h (for all patients) and 8 h (for ON1–3 patients in Table 1). The dialysate flow rate was 400 mL/min (24 L/h) for ON1–3 patients (Table 1). Part of the mixture of filtrate and discarded dialysate were collected at the rate of 1 L/h for the periods of 0–4 h and 4–8 h for ON1–3 patients. The accumulated filtrate/dialysate was mixed well before sampling.
Calculation of Estimated Aβ Influx into the Plasma
Aβ influx into the plasma during blood treatment was estimated as the sum of Aβs changed in the plasma during blood treatments and Aβs removed by dialyzers, HexDC, or both, according to the following equation.

where Aβ removed by a device during the period=(concentration of Aβ at the inlet of a device) X (removal efficiency of the device) X (plasma flow rate during the period) X (minutes of the period)
Decreased plasma concentration is denoted with a minus sign. The period is divided into 0–1, 1–4, and 4–8 h of a treatment session. Aβ concentration at the device’s inlet was set as the average of each period: 0 and 1 h for the 0–1 h period, 1 and 4 h for the 1–4 h period, and 4 and 8 h for the 4–8 h period. The removal efficiency of the devices for the 0–1 h was the removal efficiency at 1 h. The removal efficiency for the 1–4 h period was the average of those at 1 and 4 h, and the removal efficiency for the 4–8 h period was the average of those at 4 and 8 h. The whole blood volume was calculated as 1/13 of the patient’s body weight. The plasma volume was calculated as (whole blood volume) × (1 − hematocrit/100).
Measurements of Blood Concentrations of Aβs
Aβ1-40 and Aβ1-42 concentrations in the plasma were measured using the High-Sensitive Human β Amyloid (1–40) and (1–42) ELISA Kit Wako II (WAKO Pure Chemical, Osaka, Japan), respectively. ApoE4 was measured by the ApoE4/Pan-ApoE ELISA kit (MBL, Nagoya, Japan).
Statistical Analysis
All data are expressed as the mean ± standard deviation unless otherwise specified. Differences were determined using a Wilcoxon rank-sum test for nonparametric variables and the Student’s t-test for parametric variables, unless otherwise specified, using the statistical package JMP14 (SAS Institute Inc., Cary, USA). Values of p < 0.05 were considered statistically significant.
Results
Comparison of Aβ Removal Efficiencies of HexDC and PSf-HF Fragments
The in vitro Aβ removal efficiencies and reduction rates for HexDC were compared with PSf-HFs using mini-columns. Figure 3A shows the experimental circuit; quantities of HexDC and PSf-HF and the plasma flow rates were set at about 1/200 of the parameters used in clinical patient treatment. PSf-HF fragments of 2–5 nm length were packed in the column to eliminate the filtration effect and detect adsorption effects only.
Aβ1–40 and Aβ1–42 in the plasma pool were significantly decreased to about half of the initial concentrations by HexDC and PSf-HF fragment columns (solid lines in Figure 4A–D). The Aβ1–40 and Aβ1–42 concentrations at the HexDC columns’ outlet (Post) were almost zero during the treatment period, indicating that almost all Aβs flowing into the column were removed by the HexDC columns (dotted lines in Figure 4A and C). By contrast, Aβ concentrations at the outlet (Post) of PSf-HF fragment columns gradually increased after 30 min of treatment, especially the Aβ1–40 (dotted lines in Figure 4B and D). The Aβ1–40 concentrations at the outlet (Post) were similar to those in the plasma pool (Pool) at 60 min (Figure 4B), indicating that PSf-HF fragments removed almost no Aβ1-40 at the end of the treatment.
Figure 5A and B for Aβ1–40 and Figure 5C and D for Aβ1–42 show the removal efficiencies for the mini-columns and reduction rates in the plasma pool. Using HexDC, the removal efficiencies for both Aβs were approximately 100% during the entire treatment period (dashed line in Figure 5A and C). However, the removal efficiencies and reduction rates decreased after 30 min treatment using the PSf-HF fragments. The Aβ1–40 removal efficiency was significantly lower using the PSf-HF fragments than the removal efficiency using HexDC (Figure 5A, p < 0.01). The Aβ1–40 removal efficiency at 60 min using PSf-HF fragments was below zero, indicating that some adsorbed Aβ1–40 was desorbed at the end of the treatment. In contrast to the removal efficiencies, the reduction rates increased for both materials and reached more than 50% after 60 min of treatment (Figure 5B and D). Thus, more than half of the existing Aβs in the plasma pool were removed after 60 min of treatment. The Aβ1–40 reduction rates after 60 min of HexDC treatment were significantly higher than the reduction rates when using PSf-HF fragments because of the higher removal efficiency (Figure 5B, p < 0.05).
One-Pump Adsorptive Filtration Systems with PSf-HF
Adsorption on the surface of the small inner pores in the walls of hollow fibers was enhanced by filtration to increase the Aβ removal activity of PSf-HF (adsorptive filtration, Figure 3B). The previous adsorptive filtration was conducted using two pumps, a main blood circulation pump and a filtration pump through the hollow fiber walls.17 The representative clinical setting of the two-pump adsorptive filtration system is similar to that in Figure 3E, but no dialysate. A one-pump system was created to make this adsorptive filtration easy to use. The basic concept of this system was that the pressure changes in the Venturi tubes at the outlet of the dialyzer increase filtration through the hollow fibers’ membrane walls. Venturi tubes with various minimum internal diameters were produced using a 3D printer (Figure 6A and B). When melted PLA filaments were piled in the long axis direction (Figure 6C), the cross sections were not circular in parts, resulting in frequent leakage at the circuit tube connections. Melted PLA filaments were then piled circumferentially (Figure 6D), resulting in less leakage.
Circulation analysis using these Venturi tubes was first conducted using water (Figure 7A). As expected, the filtration rate (QF) depended on the tube’s narrowest inner diameter and the flow rate (QB) of water. This one-pump adsorptive filtration system was then applied to Aβ removal from human plasma with PSf hemodialyzers for 60 min (Figure 3C). The pressures before and after the hemodialyzer and at the filtrate outlet were mostly stable throughout the 60 min sessions at 50 mL/min QB (Figure 7B, an example using the Venturi tube “14j-2,” whose minimum internal diameter was 1.4 mm). The Aβ reduction rates in the plasma pool were high, as shown in Figure 7C and D for Aβ1–40 and Aβ1–42, respectively. Thus, the one-pump system functioned as designed.
Aβ Removal During Hemodialysis with Concomitant Use of HexDC and PSf Hemodialyzers
To enhance Aβ influx into the blood, an E-BARS with double Aβ removal devices seemed effective. We hypothesized that concomitant use of a HexDC column and a PSf hemodialyzer (double efficient Aβ removal devices13,14) would enhance blood Aβ removal and increase Aβ influx from the brain into the blood. Therefore, blood Aβ changes and Aβ removal efficiency were investigated in three hemodialysis patients undergoing blood purification with HexDC columns and PSf hemodialyzers (Hex-01–03 in Table 1) as an observational study. These patients underwent blood purification with HexDC columns to remove blood β2 microglobulin for carpal tunnel syndrome and hemodialysis for renal failure. Figure 3D shows the clinical circuit. During hemodialysis, excess blood water was filtered out at approximately 10 mL/min. This filtration can be considered adsorptive filtration for Aβ removal.
Figure 8A shows the plasma concentration changes for Aβ1–40 and Aβ1–42 in whole-body circulation. Both Aβ1–40 and Aβ1–42 decreased similarly. Figure 8B and C show the Aβ removal efficiencies of the HexDC column and PSf hemodialyzer, respectively. Aβ1–40 and Aβ1–42 removal efficiencies for both devices were maintained during the 4 h treatment. The removal efficiencies were lower in this clinical setting than the removal efficiencies in the in vitro experiments (Figures 3A, 5A and C). The plasma flow rates were higher in human treatment (200 mL blood/min, approximately 130 mL plasma/min) than the flow rates in in vitro experiments (equivalent to 50 mL plasma/min in humans). Lower blood flow rates resulted in higher Aβ removal efficiencies, as previously reported.14,17
Aβ Removal in Hemodialysis Patients Using PSf Hemodialyzers Alone for 8 h
Another method of enhancing Aβ influx into the blood, an E-BARS with long-time treatment, was also believed to be effective. Blood Aβ changes in hemodialysis patients using hemodialyzers alone for 4 h were already investigated in the previous study.15,16,18,19 We then hypothesized that longer hemodialysis times, such as 8 h (doubled compared with ordinal 4 h treatment), would enhance Aβ influx into the blood. Three patients suffering from end-stage renal failure underwent overnight hemodialysis for 8 h using a PSf hemodialyzer alone (ON-1–3 in Table 1). Overnight hemodialysis during sleep for 8 h was provided because these patients worked during the day. This was also an observational study. Figure 3E shows the clinical circuit. The blood was partially filtered at a 6–9 mL/min rate during hemodialysis to remove excess water, resulting in enhanced Aβ adsorption on the surface of small pores in the membrane wall (Figure 3B, adsorptive filtration).
Figure 9A shows the plasma concentration changes for Aβ1–40 and Aβ1–42 in whole-body circulation. Both Aβ1–40 and Aβ1–42 similarly decreased. The Aβ removal efficiencies using the PSf hemodialyzer were maintained at over 60% during the 8 h treatment (Figure 9B). Aβ concentrations in the filtrate of the dialyzers were also measured. The flow rate of the mixture of filtrate and waste dialysate was approximately 400 mL/min. The total volume was up to 192 L, and part of the fluid was collected at a rate of 1000 mL/h for the periods of 0–4 h and 4–8 h. Figure 9C shows the Aβ concentrations in the filtrate and waste dialysate, indicating that almost all Aβs were adsorbed (trapped) on the inner surface of the PSf-HFs.
Comparison of Aβ Influx into the Blood While Using Different Aβ Removal Systems
Removing blood Aβ evokes a large Aβ influx into the blood,15,16,18,19,22 probably from the brain.20,22 Aβ influx into the blood was estimated according to the equation shown in Figure 10 for studies of renal failure patients using the concomitant HexDC and PSf hemodialyzer for 4 h and using the hemodialyzer alone for 8 h (Figure 3D and E, respectively).
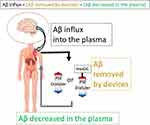 |
Figure 10 Aβ influx into the blood was calculated by subtracting the Aβ amounts decreased in the plasma from the Aβ amounts removed by the devices. |
Figure 11 shows Aβ1–40 (Figure 11A) and Aβ1–42 (Figure 11B) amounts removed by each device, and Aβs decreased in the plasma compared with the starting amounts. The Aβ amounts are shown separately for the 0–1, 1–4, and 0–4 h periods for both systems and 4–8 and 0–8 h for the dialyzer alone. Aβ1–40 removed by HexDC increased time-dependently (blue diagonal cross lattice in Figure 11A). Aβ1–40 removed by HexDC during the 1–4 h period (3 h) was nearly three times larger than the removal during the 0–1 h period. However, the Aβ1–42 removed by HexDC and both Aβ1–40 and Aβ1–42 removed by PSf dialyzers showed no clear trend over time and were suppressed in later treatment periods.
Figure 12 shows the Aβ influx evoked by the Aβ removal systems, calculated based on the data shown in Figure 11. Aβ1–40 influxes (blue diagonal striped bars in Figure 12) during concomitant use of the HexDC and PSf dialyzer were significantly (p < 0.05) higher than the influxes during use of the PSf dialyzer alone in the 1–4 h period. By contrast, Aβ1–42 influxes (red solid bars in Figure 12) during concomitant use of HexDC and the PSf dialyzer were significantly (p < 0.05) lower than influxes during the use of the PSf dialyzer alone in the 0–1, 1–4, and 0–4 h periods. Consequently, the sums of Aβ1–40 and Aβ1–42 influxes (black horizontal striped bars in Figure 12) were not significantly different between the two systems.
The average Aβ1–40 influx for both systems in the 1–4 h (3 h) period was nearly three times higher than the influx in the 0–1 h (1 h) period (11,549 ng vs 4042 ng for the concomitant use of HexDC and the PSf dialyzer and 8132 ng vs 3478 ng for PSf dialyzer alone). On the other hand, Aβ1–42 influxes in both systems during the 1–4 h period were larger than those in the 0–1 h period, but not proportional to the treatment time (425 ng vs 256 ng for the concomitant use of HexDC and PSf dialyzer, and 892 ng vs 410 ng for PSf dialyzer-alone system).
Aβ influxes for the PSf dialyzer alone during the 0–8 h period were not doubled compared with those in the 0–4 h period, as shown on the right of Figure 12. Thus, the influxes of both Aβ1–40 and Aβ1–42 may decrease over time during the treatment.
Relative Aβ Influx Normalized to the Aβ Amounts Existing in the Plasma at the Beginning of Blood Purification
The absolute amounts of Aβs removed, those decreased in systemic plasma, and Aβ influxes are dependent on the beginning plasma Aβ concentrations of each patient. Therefore, relative values for these Aβ amounts were calculated relative to the plasma Aβ amounts at the beginning of the treatment (Figure 13). Interestingly, standard deviations for each relative Aβ value were far smaller than expected. Thus, the Aβ amounts similarly changed for each patient during blood treatments.
Relative Aβ amounts removed by HexDC and PSf dialyzers (solid lines and dotted lines in Figure 13, respectively) increased time-dependently. This suggests that HexDC and PSf dialyzers had adequate Aβ adsorption capacity during the 4 and 8 h treatments. However, the changes were smaller in the latter half of the treatment than in the first half. The relative decreases in plasma Aβ (dashed lines under 0% in Figure 13) seemed to plateau after 1 h of treatment. This indicates that blood Aβ1–40 and Aβ1–42 reached homeostasis, even though large amounts of Aβ were removed from the blood because of Aβ influx.
Figure 14 shows the comparison of relative Aβ influx for the two Aβ removal systems. As shown in Figure 14A, relative Aβ1–40 influx after 4 h using the combined HexDC and PSf dialyzer was significantly higher than influx using the PSf dialyzer alone (p < 0.05). The total relative Aβ1–40 influx for the PSf dialyzer alone at 8 h was similar to that of HexDC and PSf dialyzer at 4 h. In contrast to Aβ1–40, relative Aβ1–42 influx after 4 h using the HexDC and PSf dialyzer was slightly, but significantly, lower than that of the PSf dialyzer alone (Figure 14B). A total relative Aβ1-42 influx during 8 h of treatment using the PSf dialyzer alone was almost double the Aβ1-42 influx of the combined HexDC and PSf dialyzer during 4 h.
Discussion
Regarding Aβ influx into the blood, we have reported that ordinal hemodialysis with hemodialyzers alone for 4 h evoked an influx of 7219 ng Aβ1–40 and 664 ng Aβ1–42 based on analysis of 30 hemodialysis patients.19 In this study, as shown in Figure 12, Aβ1–40, Aβ1–42, Aβ1–40 + Aβ1–42 influx evoked by the concomitant use of HexDC and the PSf hemodialyzer for 0–4 h were 15,591 ± 5407 ng, 690 ± 166 ng, and 16,281 ± 5565 ng, respectively. Those by PSf hemodialyzer alone were 11,610 ± 1335 ng, 1302 ± 211 ng, and 12,912 ± 1224 ng for 0–4 h, and 19,198 ± 1556 ng, 1673 ± 928 ng, and 20,871 ± 1699 ng for 0–8 h, respectively. Thus, compared with E-BARS with hemodialyzers alone for 4 h, E-BARS with double devices of Aβ removal (HexDC and PSf hemodialyzer) for 4 h evoked nearly doubled Aβs (Aβ1–40 + Aβ1–42) influx: 7219 ng vs 16,281 ng, and E-BARS of longer treatment with PSf hemodialyzer alone for 8 h also evoked more than doubled or nearly tripled Aβs (Aβ1–40 + Aβ1–42) influx: 7219 ng vs 20,871 ng. Aβ influx in this study was two to three times larger than the estimated total Aβs (7760 ng) in the normal human brain28 and far larger than Aβs removed by peritoneal dialysis: 131.33 ng, which was approximately 2% of the total Aβ in the normal brain.25 Therefore, efficient Aβ removal systems, such as a combination of HexDC and PSf hemodialyzer or longer treatment time, may be more effective in removing brain Aβs than ordinal hemodialysis (hemodialyzers alone) for 4 h.
One aim of our blood Aβ removal systems was to enhance Aβ influx (migration) from the brain into the blood, especially Aβ1–42, which readily forms neurotoxic Aβ1–42 oligomers. Both Aβ removal systems in this study showed very similar time dependency (Figure 14B) for relative Aβ1–42 influxes. However, PSf dialyzer alone evoked slightly higher (p < 0.05) relative Aβ1–42 influx than the combined system of HexDC and PSf dialyzer. Furthermore, the 8 h treatment with the PSf hemodialyzer alone evoked significantly larger, almost doubled, Aβ1–42 influx than the concomitant use of HexDC and the PSf hemodialyzer for 4 h (Figure 14B). Focused on Aβ1–42 removal, PSf hemodialyzer alone may have enough ability to evoke Aβ1–42 influx into the blood.
In contrast to the Aβ1–42 influx, concomitant use of HexDC and the PSf hemodialyzer evoked a larger Aβ1–40 influx (significantly for only 1–4 h) than the PSf hemodialyzer alone (Figures 12 and 14A).
Overall, Aβ removal systems with concomitant use of HexDC and PSf hemodialyzer evoked similar or less Aβ1–42 influxes and larger Aβ1–40 influx compared with Aβ influx using the PSf hemodialyzer alone. Although the reason for this difference is unclear at present, it provides information to establish appropriate systems of E-BARS.
Regarding discrepancy between this and previous studies, Aβ influx with PSf hemodialyzer in 0–4 h period at night in this study was larger than those we reported previously for ordinal hemodialysis patients for 4 h during the day:19 12,912 ng vs 16,281 ng. One possible reason is the variations in hemodialyzer materials used. Hemodialyzer material in this study was only PSf, one of the efficient Aβ adsorbents. By contrast, some hemodialyzers used in the previous study included less efficient Aβ removal devices, such as cellulose triacetate. Another reason may be that blood purification was conducted overnight or daytime. In this study, the blood purification with the PSf hemodialyzer alone for 8 h was provided overnight, including when patients were sleeping, whereas 4 h blood purification was provided during the day in the previous study.19 It was reported that Aβ concentrations in CSF are higher in the evening (about 18:00–22:00) than during the awake daytime hours and decrease while a patient is sleeping (about 22:00–7:00).29,30 Therefore, the differences in Aβs concentrations in CSF depending on the time of day might affect this study’s results. Thus, blood Aβ removal during sleeping might be an effective method for brain Aβ removal.
The amounts of Aβ1–40 and Aβ1–42 removed by the PSf hemodialyzers in concomitant use with HexDC were smaller than the Aβ amounts removed by PSf hemodialyzers alone in each period of 0–1, 1–4, and 0–4 h, as shown in Figure 11A and B (the solid orange bars of “HexDC + Dialyzer” and “Dialyzer only”). One explanation is that the Aβ concentrations at the PSf hemodialyzer inlet (Figure 3D) were lower than the Aβ concentrations for the PSf hemodialyzer directly connected with the blood vessels of patients (Figure 3E) because approximately 50–60% of the Aβs in the blood were already removed by the HexDC column (Figure 3D) before PSf hemodialyzer in the system of concomitant use of “tandemly” connected HexDC and PSf hemodialyzer. To use the total Aβ removal activity capacity of PSf hemodialyzer, blood should be introduced “in parallel” to the HexDC and PSf hemodialyzer systems. However, such parallel systems require doubled blood flow rates, which are unsuitable for Japanese hemodialysis patients because of blood outflow limitations.
Figure 15 summarizes the factors that may affect blood Aβ removal. The key factors are not only Aβ removal efficacy of the device but also the treatment period, blood flow rates into the devices, Aβ concentrations in the blood, Aβ concentrations in CSF that change depending on the time of day and Aβ production/degradation rates in the brain, the activity of Aβ transporters (for example, LRP-1 and RAGE), and frequency of treatment (for example, three times a week, or once a month?) of Aβ removal. When comparing dual Aβ removal devices with a single Aβ removal device, the relative Aβ1–42 influx was similar (Figure 14B). Therefore, the rate of Aβ influx to the blood from the brain, especially Aβ1–42, might be the rate-limiting step for brain Aβ1–42 removal, even if more effective devices are used for Aβ removal.
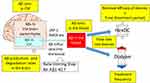 |
Figure 15 Aβ transportation pathway from the brain to Aβ removal devices. Key factors affecting Aβ influxes and removal are also shown. |
Conclusions
As blood Aβ removal devices, PSf hemodialyzer in adsorptive filtration methods and HexDC showed comparable adsorption capacity for plasma Aβs. Doubled Aβ removal devices consisting of tandemly connected HexDC and PSf hemodialyzer for 4 h evoked a larger Aβ1–40 influx into the blood than PSf hemodialyzer alone for the same period. However, both systems evoked similar Aβ1–42 influx for 4 h. These systems evoked Aβs (Aβ1–40 + Aβ1–42) influx nearly two times larger than Aβs existing in the normal brain. Furthermore, longer Aβ removal evoked more influxes of Aβ1–40 and Aβ1–42. These findings of blood Aβ removal systems may lead to an optimal clinical setting for therapy and prevention of AD.
Acknowledgments
The authors thank Hiroshi Tomizawa of Mizuno Clinic, and, Yuri Sakakibara, Kota Watanabe, Miki Kamiya, and Tatsuya Hama of Fujita Health University for their technical assistance. The authors also thank Yoshiyuki Hiki for fruitful discussion, and Fumiyasu Hirai and Ai Yonezawa of Kaneka for providing HexDC for in vitro experiments. This work was partly supported by KAKENHI (20509008, 23500531, 26282126) and the Smoking Research Foundation.
Disclosure
Nobuya Kitaguchi has stock ownership in Asahi Kasei Corporation Co., Ltd and reports grants from Japanese Government, grants from Smoking Research Foundation, grants from Asahi Kasei Medical Co. LTD, grants, non-financial support from Kaneka Corporation, during the conduct of the study.
The other authors declare that they have no conflict of interest.
References
1. Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi:10.1152/physrev.2001.81.2.741
2. Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi:10.1126/science.1197623
3. Aisen PS, Cummings J, Doody R, et al. The future of anti-amyloid trials. J Prev Alzheimers Dis. 2020;7:146–151. doi:10.14283/jpad.2020.24
4. Selkoe DJ. A is for amyloid. J Prev Alz Dis. 2020;3:140–141. doi:10.14283/jpad.2020.27
5. In DIAN-TU, gantenerumab brings down tau. By a Lot. Open Extension Planned. Alzforum; 2020. Available from: https://www.alzforum.org/news/conference-coverage/dian-tu-gantenerumab-brings-down-tau-lot-open-extension-planned.
6. Kawaguchi K, Kitaguchi N, Nakai S, et al. Novel therapeutic approach for Alzheimer’s disease by removing amyloid beta protein from the brain with an extracorporeal removal system. J Artif Organs. 2010;13:31–37. doi:10.1007/s10047-010-0482-3
7. Schoonenboom NS, Mulder C, Van Kamp GJ, et al. Amyloid beta 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol. 2005;58:139–142. doi:10.1002/ana.20508
8. Lopez OL, Kuller LH, Mehta PD, et al. Plasma amyloid levels and the risk of AD in normal subjects in the cardiovascular health study. Neurology. 2008;70:1664–1671. doi:10.1212/01.wnl.0000306696.82017.66
9. Bell RD, Sagare AP, Friedman AE, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi:10.1038/sj.jcbfm.9600419
10. Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi:10.1007/s00401-006-0115-3
11. Silverberg GD, Miller MC, Messier AA, et al. Amyloid deposition and influx transporter expression at the blood–brain barrier increase in normal aging. J Neuropathol Exp Neurol. 2010;69:98–108. doi:10.1097/NEN.0b013e3181c8ad2f
12. Lao K, Zhang R, Luan J, et al. Therapeutic strategies targeting amyloid-β receptors and transporters in Alzheimer’s disease. J Alzheimers Dis. 2021;79:1429–1442. doi:10.3233/JAD-200851
13. Kawaguchi K, Takeuchi M, Yamagawa H, et al. A potential therapeutic system for Alzheimer’s disease using adsorbents with alkyl ligands for removal of blood Amyloid β. J Artif Organs. 2013;16:211–217. doi:10.1007/s10047-012-0675-z
14. Kawaguchi K, Saigusa A, Yamada S, et al. Toward the treatment for Alzheimer’s disease: adsorption is primary mechanism of removing amyloid β protein with hollow-fiber dialyzers of the suitable materials, polysulfone and polymethyl methacrylate. J Artif Organs. 2016;19:149–158. doi:10.1007/s10047-015-0878-1
15. Kitaguchi N, Kawaguchi K, Nakai S, et al. Reduction of Alzheimer’s disease Amyloid-β in plasma by hemodialysis and its relation to cognitive functions. Blood Purif. 2011;32:57–62. doi:10.1159/000322624
16. Kato M, Kawaguchi K, Nakai S, et al. Potential therapeutic system for Alzheimer’s disease: removal of blood Aβs by hemodialyzers and its effect on the cognitive functions of renal-failure patients. J Neural Transm (Vienna). 2012;119:1533–1544. doi:10.1007/s00702-012-0844-5
17. Kitaguchi N, Kawaguchi K, Yamazaki K, et al. Adsorptive filtration systems for effective removal of blood amyloid β: a potential therapy for Alzheimer’s disease. J Artif Organs. 2018;21:220–229. doi:10.1007/s10047-017-1012-3
18. Kitaguchi N, Hasegawa M, Ito S, et al. A prospective study on blood Aβ levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer’s disease. J Neural Transm (Vienna). 2015;122:1593–1607. doi:10.1007/s00702-015-1431-3
19. Kitaguchi N, Tatebe H, Sakai K, et al. Influx of tau and amyloid-β proteins into the blood during hemodialysis as a therapeutic extracorporeal blood Aβ removal system for Alzheimer’s disease. J Alzheimers Dis. 2019;69:687–707. doi:10.3233/JAD-190087
20. Sakai K, Senda T, Hata R, et al. Patients that have undergone hemodialysis exhibit lower amyloid deposition in the brain: evidence supporting a therapeutic strategy for Alzheimer’s disease by removal of blood amyloid. J Alzheimers Dis. 2016;51:997–1002. doi:10.3233/JAD-151139
21. Kitaguchi N, Kato T, Matsunaga S, et al. Removal of blood amyloid-β with hemodialysis reduced brain amyloid-β, confirmed by brain imaging: a case report. Neuropsychiatr Dis Treat. 2018;14:2931–2937. doi:10.2147/NDT.S186118
22. Kitaguchi N, Kawaguchi K, Kinomura J, et al. [P2-042]: extracorporeal blood Aβ removal system (EBARS) reduced soluble Aβ in the brain by triggering influx into the blood: rat studies. Alzheimers Dement. 2017;13:P620–P621. doi:10.1016/j.jalz.2017.06.690
23. Nakai S, Wakai K, Kanda E, et al. Is hemodialysis itself a risk factor for dementia? An analysis of nationwide registry data of patients on maintenance hemodialysis in Japan. Ren Replace Ther. 2018:4. doi:10.1186/s41100-018-0154-y.
24. Saito Y, Sakata M, Kobayakawa M, et al. Removal of Aβ oligomers from the blood: a potential therapeutic system for Alzheimer’s disease. Neuropsychiatr Dis Treat. 2020;16:607–627. doi:10.2147/NDT.S241074
25. Jin WS, Shen LL, Bu XL, et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017;134:207–220. doi:10.1007/s00401-017-1721-y
26. Boada M, López O, Olazarán J, et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: primary results of the AMBAR Study. Alzheimers Dement. 2020;16:1412–1425. doi:10.1002/alz.12137
27. Wood H. Alzheimer disease: peripheral Aβ clearance-a therapeutic strategy for AD? Nat Rev Neurol. 2017;13:386. doi:10.1038/nrneurol.2017.80
28. Roberts KF, Elbert DL, Kasten TP, et al. Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol. 2014;76:837–844. doi:10.1002/ana.24270
29. Huang Y, Potter R, Sigurdson W, et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch Neurol. 2012;69:51–58. doi:10.1001/archneurol.2011.235
30. Kang JE, Lim MM, Bateman RJ, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi:10.1126/science.1180962
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.