Back to Journals » Risk Management and Healthcare Policy » Volume 16
A High De Ritis Ratio is Associated with Mortality in Adult Trauma Patients
Authors Tsai CH, Hsieh TM, Hsu SY, Hsieh CH
Received 1 March 2023
Accepted for publication 9 May 2023
Published 12 May 2023 Volume 2023:16 Pages 879—887
DOI https://doi.org/10.2147/RMHP.S409345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Haiyan Qu
Ching-Hua Tsai, Ting-Min Hsieh, Shiun-Yuan Hsu, Ching-Hua Hsieh
Department of Trauma Surgery, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, 83301, Taiwan
Correspondence: Ching-Hua Hsieh, Tel +886-7-7327476, Email [email protected]
Introduction: The De Ritis ratio, which is the ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT), has been suggested as a potential prognostic marker for various diseases. This study aimed to investigate the association between the De Ritis ratio and in-hospital mortality in adult trauma patients.
Methods: A total of 17,472 adult trauma patients hospitalized between January 1, 2009, and December 31, 2020, were allocated into groups according to the De Ritis ratio. The normal range of the De Ritis ratio was calculated from 3320 individuals in the National Taiwan Biobank. Statistical analyses were performed using SPSS software.
Results: Patients with a De Ritis ratio > 1.6 had a significantly higher in-hospital mortality rate (7.3% vs 1.5%, odds ratio 5.29; Q1–Q3 2.72– 10.30; p < 0.001) and a 2.71-fold higher in-hospital mortality rate (Q1–Q3 1.24– 5.92; p = 0.012), after adjusting for sex, age, comorbidities, consciousness level, and injury severity, than those with a De Ritis ratio within the reference values.
Discussion: This study revealed that a De Ritis ratio > 1.6 may serve as an early prognostic tool to identify adult trauma patients at high risk of in-hospital mortality.
Keywords: aspartate aminotransferase, AST, alanine aminotransferase, ALT, De Ritis ratio, in-hospital mortality, trauma
Introduction
The liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are commonly used to assess liver activity in individuals with different liver disorders.1–4 The majority of ALT is located in the cytoplasm of hepatocytes, and an elevated blood level of ALT suggests parenchymal liver disease with liver-specific malfunction. AST, on the other hand, is found in tissues other than the liver, including the brain, heart, kidneys, and skeletal muscle.5 Changes in AST levels are involved in systemic disorders other than parenchymal liver disease, such as ischemia-reperfusion injury, increased oxidative stress, and metabolic disorder.6–8
Because ALT levels particularly suggest parenchymal liver disease, whereas AST levels are associated with various organs and thus impacted by numerous diseases, variations in these two enzymes may differ between diseases.5 Fernando De Ritis suggested the De Ritis ratio, a measure of blood AST and ALT values, for the detection of viral hepatitis in 1957.9 In addition, this ratio has been shown to be helpful in distinguishing between diverse sources of liver disease5,10,11 and is an important predictive factor in the management of a variety of cancer12–15 and non-malignant illnesses.6–8 The De Ritis ratio may be a proxy measure for ischemia end-organ damage16 and offers useful information for risk evaluations of patients with cardiovascular disease,16–18 cardiac failure,19 acute renal injury,20–22 sepsis,23 and even COVID-19.24–27
Although the ratio of ALT to other liver-related enzymes including alkaline phosphatase, gamma glutamyl transpeptidase, and glutamate dehydrogenase has been extensively studied, the De Ritis ratio is the only enzyme ratio that has withstood the test of time and is still widely used in clinical settings.5 However, the De Ritis ratio has not been studied in patients with trauma injuries, with the exception of two studies showing its utility in forecasting mortality in patients with major burn injuries28 and after burn surgery29. The objective of this observational research was to determine the relationship between the De Ritis ratio at admission and the results of admitted trauma patients using a database from a trauma registry. A large-scale community-based sample from the Taiwan Biobank, the biggest national biobank in Taiwan30 was used to determine the usual range of the De Ritis ratio. This research showed that a De Ritis ratio greater than or equal to 1.60 may be used as a predictive instrument to identify adult trauma patients with a high in-hospital mortality risk.
Materials and Methods
Population Research and Data Gathering
First, a study was conducted to establish the typical range of the De Ritis ratio using de-identified health data of adult patients from a large-scale community-based sample from Taiwan Biobank, the biggest national biobank in Taiwan. The Taiwan Biobank is managed by the Taiwanese government in order to attract Taiwanese participants and provide chances for joint study to primary scientists.30,31 Each Taiwan Biobank member completed an authorized informed consent document, and data was gathered in accordance with applicable government laws.31 From Oct 24, 2012 till June 30, 2022, 172,078 equally dispersed voluntary donors from northern, southern, and eastern Taiwan, correspondingly, were recruited. We requested the data of 3400 participants based on the criterion of randomly selected individuals having blood laboratory data, with a male-to-female ratio of 1:1 and the exclusion of malignant patients. The blood values of AST and ALT, as well as the calculated De Ritis ratio, were gathered from 3320 Taiwan Biobank participants. The 95% confidence interval (CI) of the De Ritis ratio of normal individuals from the biobank served as the standard for selecting the control group from the trauma patient cohort. Before the research was conducted, Chang Gung Memorial Hospital’s Institutional Review Board (IRB) authorized the procedure (approval numbers 201800396B0 and 202100842B0). The necessity for specific patient permission was removed due to the retrospective nature of this research, in accordance with the IRB and Taiwan Biobank rule.
In addition, we included admitted adult patients (equal or more than 20 years) with all types of trauma who were registered in the hospital’s Trauma Registration System in a single trauma center32–34 between January 1, 2009 and December 31, 2020 (Figure 1). After excluding patients who lacked AST or ALT data (n = 19,295) and those with burns (n = 981), hanging injuries (n = 17), and who drowned (n = 2), there were 17,472 patients with traumatic injuries in the study population. Patients’ medical information was obtained from the hospital’s trauma registry, including sex, age, preexisting comorbidities (diabetes mellitus [DM], hypertension [HTN], coronary artery disease [CAD], cerebrovascular accident [CVA], congestive heart failure [CHF], and end-stage renal disease [ESRD]), serum AST and ALT levels (U/L) upon arrival at the emergency room, derived De Ritis ratio, the conscious level of Glasgow Coma Scale (GCS) score, the injury severity by Injury Severity Score (ISS), and in-hospital mortality. The research population was divided into groups of patients with a De Ritis ratio between 1.18–1.21 (n = 616), < 1.18 (n = 5861), and >1.21 (n = 10,995), following the selection of the control group (which had a mean De Ritis ratio of 1.20 and a 95% confidence interval of 1.18–1.21) as described earlier. The latter two divisions were split into categories based on the value of each decline or rise, using periods of about 0.2.
Statistical Analyses
For all statistical studies, Windows SPSS statistical software (version 23.0; IBM Inc., Chicago, IL, USA) was utilized. Categorical data were analyzed using two-sided Fisher’s exact test. Using the Kolmogorov–Smirnov test, the normalization of the continuously dispersed factors was assessed. Analysis of variance with Bonferroni post-hoc adjustment was used for normally distributed continuous data, while the Mann–Whitney U-test was used for non-normally distributed continuous data. Continuous and non-continuous data are presented as mean with standard deviation or median with interquartile range, respectively (IQR; Q1–Q3). Mortality within the hospital was the main result of this research. The odds ratio (ORs) of death was computed using a 95% confidence range. The primary outcome of this study is in-hospital mortality. Using logistic regression, the adjusted odds ratio (AOR) of death was determined, adjusting for factors with substantial variations in patient injury features. The log-rank (Mantel-Cox) test and Kaplan-Meier curves were used to evaluate the statistical significance of the time to mortality between patients with high De Ritis ratio vs those within the reference De Ritis ratio. In all studies, a two-tailed p value 0.05 was deemed significant.
Results
De Ritis Ratio of the Patients in the Taiwan Biobank
1330 (40.1%) of the 3320 sample Taiwan Biobank respondents were male, while 1990 (59.9%) were female. The average age of the patients was 57.6 ± 7.10 years (mean ± SD). The mean serum AST and ALT values were 25.7 ± 12.8 U/L and 24.7 ± 18.9 U/L, respectively, and the mean De Ritis ratio was 1.20 ± 0.43 (mean ± SD). These individuals’ 95% confidence interval for the De Ritis ratio was 1.18–1.21, which was used to reflect the De Ritis ratio of the overall community.
Outcomes of the Trauma Patients According to Stratified De Ritis Ratio
As shown in Table 1, 1.5% (9/616) of individuals with a De Ritis ratio between 1.18 and 1.21 died. There was no statistically significant variation in the death rate between the groups of individuals with De Ritis ratios between 1.21 and 1.4 and between 1.4 and 1.6. Those with a De Ritis ratio >1.6 had a substantially increased in-hospital mortality rate, and those with a larger De Ritis ratio had a higher risk of in-hospital mortality (Figure 2). Those with a De Ritis ratio between 2.0 and 2.2 had a 5.35-fold (95% CI 2.61 to 10.94) greater risk of in-hospital mortality compared to those within the standard De Ritis ratio, whereas those with a De Ritis ratio >3 had a 12.10-fold (95% CI 5.94 to 24.67) higher risk of in-hospital mortality.
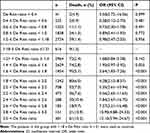 |
Table 1 The Study Population Groups According to De Ritis Ratio and the Associated Odds of In-hospital Mortality Compared to Those Within the Reference De Ritis Ratio (1.18–1.21) |
Outcomes of Patients with a De Ritis Ratio >1.6
To minimize the impact of initial patient traits on the outcome assessment, we computed the adjusted in-hospital mortality rate of patients with a De Ritis ratio >1.6 versus those with a De Ritis ratio between 1.18 and 1.21. Patients with a De Ritis ratio greater than 1.6 were more likely to be female, were older, and had higher AST levels but lower ALT levels than those within the reference De Ritis ratio (Table 2). The rates of pre-existing conditions did not vary significantly, with the exception of CAD, which was greater in patients with a De Ritis ratio >1.6 than in patients within the standard De Ritis ratio. Patients with a De Ritis ratio >1.6 had a significantly lower GCS (median [IQR, Q3-Q3]: 15 [14–15] vs 15 [15–15], respectively; p < 0.001) but a higher ISS (9 [8–17] vs 9 [4–13]; p < 0.001) than those within the reference De Ritis ratio. Compared to individuals with a De Ritis ratio within the reference range, those with a ratio larger than 1.6 had a much higher death rate (7.3% vs 1.5%, OR: 5.29, Q1-Q3: 2.72–10.30; p < 0.001) and an adjusted in-hospital mortality rate (AOR: 2.71, Q1-Q3: 1.24–5.92; p = 0.012). This difference in the time to mortality was statistically significant between the groups of patients with a ratio larger than 1.6 and those with a ratio within the reference range (p < 0.001) on applying Log Rank (Mantel-Cox) analysis (Figure 3).
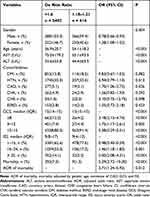 |
Table 2 The Injury and Patient Characteristics of Patients with a De Ritis Ratio >1.6 vs Those Within the Reference De Ritis Ratio (1.18–1.21) |
Discussion
This research showed that among adult trauma patients, those with a De Ritis ratio >1.6 had a substantially increased in-hospital mortality rate, and a greater De Ritis ratio was associated with an increased likelihood of death. Even after accounting for the variance in initial features of the research group, the adjusted death rate of patients with a De Ritis ratio >1.6 was 2.71 times that of those with a De Ritis ratio within the standard values. Hence, the De Ritis ratio is applicable not just to patients with liver injuries, but also to patients with other forms of trauma injuries. If the De Ritis ratio is more than 1.6 in adult trauma patients who have been injured by any type of trauma, this should serve as a warning indication that the patient is at an increased risk for in-hospital mortality.
For patients with liver damage, hepatocyte loss is raised above typical baseline values, with AST elevated at a rate greater than twofold that of ALT.35 However, our Trauma Registration System had 1.54% of trauma victims with liver injury.36 Notably, AST and ALT play important roles in carbohydrate and protein metabolism, which is a component of the malate-aspartate shuttle pathway that allows NADH/NAD+ conversion2 and is involved in aerobic glycolysis, whereas ALT plays a crucial role in the glucose-alanine cycle to produce glucose to meet glucose requirements.5 Due to the fact that AST and ALT activate nucleotides and non-essential amino acids engaged in aerobic glycolysis and boost glutamine metabolism in growing cancer cells,37–40 the De Ritis ratio is associated with tumor metabolism in numerous tumors that utilize glucose.41 Therefore, we propose that an increased De Ritis ratio represents the need for glucose intake in trauma patients experiencing stress. Given that a multifactorial mechanism is more likely to account for the higher De Ritis ratio, the idea of increased glucose uptake constitutes one of the potential mechanisms that will require more investigation in future studies.
There is currently no consensus on the reference ranges for the De Ritis ratio. In this research, the normal range of the De Ritis ratio was computed based on the normal population in a national biobank, and a De Ritis ratio of 1.6 was identified as the benchmark for identifying trauma patients with a greater chance of death. Clearly, the threshold number of the De Ritis ratio can differ significantly based on disease. For instance, among heart valve replacement surgery patients, a De Ritis ratio ≥ 1.19 on entry was substantially associated with bleeding propensity under warfarin treatment.42 A De Ritis ratio ≥ 1.2 suggested an increased risk for patients with acute myocardial infarction16 and forecasted both cancer-specific survival and total survival outcomes in patients treated for advanced renal cell carcinoma.43 A De Ritis ratio ≥ 1.35 was a meaningful predictive indicator for chemotherapy-treated individuals with advanced prostate cancer.44 A De Ritis ratio ≥ 1.5 is a major predictive factor for renal cell cancer patients undergoing surgery.45 Following surgery, a threshold De Ritis ratio of 1.6 was found for overall and cancer-specific mortality in individuals with urothelial carcinoma of the upper urinary system.46 Patients with peripheral vascular occlusive disease who had a De Ritis ratio greater than 1.67 had a twofold increased risk of developing critical limb ischemia.47 As a predictive indicator for individuals with alcoholic hepatitis48,49 and distal cholangiocarcinoma,50 a De Ritis ratio > 2.0 was utilized.
This research has several limitations. To begin, the way the retrospective study was set up could have introduced selection bias. Furthermore, a possible selection bias in the outcome assessment was introduced by measuring in-hospital mortality but not long-term mortality, since the latter is not present in the coded trauma database. In addition, the removal of individuals without entry AST and ALT values may have led to selection bias. Another disadvantage is the lack of time between the injury and the initial AST/ALT measurements in the hospital, given that De Ritis ratio temporal variations among individual individuals might vary greatly. In addition, a patient’s De Ritis ratio may also change due to the existence of an undetected liver disease, the use of drugs that affect AST or ALT values, or even life-saving interventions like cardiopulmonary resuscitation (CPR). Since this study only included patients at one urban trauma center, its results may have limited relevance outside of that institution.
Conclusions
This research showed that a De Ritis ratio > 1.6 may be used as a predictive indicator to identify adult trauma patients at high risk for in-hospital mortality.
Data Sharing Statement
Data available on request.
Acknowledgments
We value the Biostatistics Center’s help with statistical studies at Kaohsiung Chang Gung Memorial Hospital.
Funding
This study was supported by Chang Gung Memorial Hospital Grant No. CMRPG8J0981.
Disclosure
The authors declare that they have no competing interests.
References
1. Moriles KE, Azer SA. Alanine amino transferase. In: StatPearls. StatPearls Publishing, StatPearls Publishing LLC; 2022.
2. Otto-ślusarczyk D, Graboń W, Mielczarek-Puta M. Aminotransferaza asparaginianowa--kluczowy enzym w metabolizmie ogólnoustrojowym człowieka [Aspartate aminotransferase--key enzyme in the human systemic metabolism]. Postepy Hig Med Dosw. 2016;70:219–230. Polish. doi:10.5604/17322693.1197373
3. Suciu A, Abenavoli L, Pellicano R, Luzza F, Dumitrascu DL. Transaminases: oldies but goldies. A narrative review. Minerva Gastroenterol Dietol. 2020;66(3):246–251. doi:10.23736/s1121-421x.20.02660-4
4. Sharpe PC. Biochemical detection and monitoring of alcohol abuse and abstinence. Ann Clin Biochem. 2001;38(Pt 6):652–664. doi:10.1258/0004563011901064
5. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117–130.
6. Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. 2015;21(3):711–725. doi:10.3748/wjg.v21.i3.711
7. Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol. 2012;18(29):3775–3781. doi:10.3748/wjg.v18.i29.3775
8. Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082–8091. doi:10.3748/wjg.v20.i25.8082
9. De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: the transaminase serum activities. 1957. Clin Chim Acta. 2006;369(2):148–152. doi:10.1016/j.cca.2006.05.001
10. Mo Q, Liu Y, Zhou Z, et al. Prognostic value of aspartate transaminase/alanine transaminase ratio in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy. Front Oncol. 2022;12:876900. doi:10.3389/fonc.2022.876900
11. Darstein F, Häuser F, Straub BK, et al. Hepatitis E virus genotype 3 is a common finding in liver-transplanted patients undergoing liver biopsy for elevated liver enzymes with a low De Ritis ratio and suspected acute rejection: a real-world cohort. Clin Transplant. 2018;32(11):e13411. doi:10.1111/ctr.13411
12. Ghahari M, Salari A, Ghafoori Yazdi M, et al. Association between preoperative De Ritis (AST/ALT) ratio and oncological outcomes following radical cystectomy in patients with urothelial bladder cancer. Clin Genitourin Cancer. 2022;20(2):e89–e93. doi:10.1016/j.clgc.2021.10.007
13. Fukui-Kawaura S, Kawahara T, Araki Y, et al. A higher De Ritis ratio (AST/ALT) is a risk factor for progression in high-risk non-muscle invasive bladder cancer. Oncotarget. 2021;12(9):917–922. doi:10.18632/oncotarget.27944
14. Janisch F, Klotzbücher T, Marks P, et al. Predictive value of De Ritis ratio in metastatic renal cell carcinoma treated with tyrosine-kinase inhibitors. World J Urol. 2021;39(8):2977–2985. doi:10.1007/s00345-021-03628-2
15. Olcucu MT, Karamik K, Yilmaz K, Okuducu Y, Cakir S, Ates M. Preoperative inflammation markers and De Ritis Ratio in predicting clinical presentation and prognosis of patients with testicular germ cell tumors. J Coll Physicians Surg Pak. 2020;30(10):1041–1046. doi:10.29271/jcpsp.2020.10.1041
16. Steininger M, Winter MP, Reiberger T, et al. De-Ritis ratio improves long-term risk prediction after acute myocardial infarction. J Clin Med. 2018;7(12):474. doi:10.3390/jcm7120474
17. Jasiewicz M, Siedlaczek M, Kasprzak M, et al. Elevated serum transaminases in patients with acute coronary syndromes: do we need a revision of exclusion criteria for clinical trials? Cardiol J. 2021. doi:10.5603/CJ.a2021.0081
18. Djakpo DK, Wang ZQ, Shrestha M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch Med Sci Atheroscler Dis. 2020;5:e279–e283. doi:10.5114/amsad.2020.103028
19. Lu Z, Ma G, Chen L, Devaux Y. De-Ritis ratio is associated with mortality after cardiac arrest. Dis Markers. 2020;2020:8826318. doi:10.1155/2020/8826318
20. Pilarczyk K, Carstens H, Heckmann J, et al. The aspartate transaminase/alanine transaminase (DeRitis) ratio predicts mid-term mortality and renal and respiratory dysfunction after left ventricular assist device implantation. Eur J Cardiothorac Surg. 2017;52(4):781–788. doi:10.1093/ejcts/ezx247
21. Park JY, Yu J, Hong JH, et al. Elevated De Ritis ratio as a predictor for acute kidney injury after radical retropubic prostatectomy. J Pers Med. 2021;11(9):836. doi:10.3390/jpm11090836
22. He HM, He C, Zhang SC, et al. Predictive value of aspartate aminotransferase-to-alanine aminotransferase ratio for contrast-associated acute kidney injury in patients undergoing elective percutaneous coronary intervention. J Cardiol. 2022;79(5):618–625. doi:10.1016/j.jjcc.2021.11.009
23. Zhao PY, Yao RQ, Ren C, et al. De Ritis ratio as a significant prognostic factor in patients with sepsis: a retrospective analysis. J Surg Res. 2021;264:375–385. doi:10.1016/j.jss.2021.03.018
24. Zinellu A, Arru F, De Vito A, et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest. 2021;51(1):e13427. doi:10.1111/eci.13427
25. Pranata R, Huang I, Lim MA, et al. Elevated De Ritis ratio is associated with poor prognosis in COVID-19: a systematic review and meta-analysis. Front Med. 2021;8:676581. doi:10.3389/fmed.2021.676581
26. Guzey-Aras Y, Yazar H, Acar T, et al. The role of De Ritis ratio as a clinical prognostic parameter in COVID 19 Patients. Clin Lab. 2021;67(10). doi:10.7754/Clin.Lab.2021.210119
27. Yashashwini A, Vedavathi R. The study of De Ritis (Ast/ Alt) Ratio in comparision with other parameters for predicting poor prognosis in Covid 19 patients. J Assoc Physicians India. 2022;70(4):11–12.
28. Wang B, Hu L, Chen Y, et al. Aspartate transaminase/alanine transaminase (De Ritis ratio) predicts survival in major burn patients. Burns. 2022;48(4):872–879. doi:10.1016/j.burns.2021.08.006
29. Yu J, Kim HY, Kong YG, Park JH, Seo YJ, Kim YK. De Ritis ratio as a predictor of 1-year mortality after burn surgery. Burns. 2021;47(8):1865–1872. doi:10.1016/j.burns.2021.02.001
30. Fan CT, Lin JC, Lee CH. Taiwan Biobank: a project aiming to aid Taiwan’s transition into a biomedical island. Pharmacogenomics. 2008;9(2):235–246. doi:10.2217/14622416.9.2.235
31. Fan CT, Hung TH, Yeh CK. Taiwan Regulation of Biobanks. J Law Med Ethics. 2015;43(4):816–826. doi:10.1111/jlme.12322
32. Hsieh CH, Hsu SY, Hsieh HY, Chen YC. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed J. 2017;40(2):113–120. doi:10.1016/j.bj.2016.10.005
33. Hsieh CH, Liu HT, Hsu SY, Hsieh HY, Chen YC. Motorcycle-related hospitalizations of the elderly. Biomed J. 2017;40(2):121–128. doi:10.1016/j.bj.2016.10.006
34. Hsieh CH, Chen YC, Hsu SY, Hsieh HY, Chien PC. Defining polytrauma by abbreviated injury scale >/= 3 for a least two body regions is insufficient in terms of short-term outcome: a cross-sectional study at a level I trauma center. Biomed J. 2018;41(5):321–327. doi:10.1016/j.bj.2018.08.007
35. Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi:10.1016/s0016-5085(03)00907-7
36. Chou SE, Rau CS, Su WT, Tsai CH, Hsu SY, Hsieh CH. The Association of Albumin-Bilirubin (ALBI) grade with mortality risk in trauma patients with liver injuries. Risk Manag Healthc Policy. 2023;16:279–286. doi:10.2147/rmhp.S397210
37. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi:10.1038/nrc2981
38. Elf SE, Chen J. Targeting glucose metabolism in patients with cancer. Cancer. 2014;120(6):774–780. doi:10.1002/cncr.28501
39. Lin LY, Hsu CY, Chiou HY, et al. Association between dietary patterns and serum hepatic enzyme levels in adults with dyslipidemia and impaired fasting plasma glucose. Nutrients. 2021;13(3). doi:10.3390/nu13030987
40. Colomba J, Netedu SR, Lehoux-Dubois C, et al. Hepatic enzyme ALT as a marker of glucose abnormality in men with cystic fibrosis. PLoS One. 2019;14(7):e0219855. doi:10.1371/journal.pone.0219855
41. Tai YS, Chen CH, Huang CY, Tai HC, Wang SM, Pu YS. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab Res Rev. 2015;31(3):307–314. doi:10.1002/dmrr.2614
42. Cao Y, Chen G, Li H, et al. De Ritis ratio as a significant prognostic factor of international normalized ratio ≥4 in the initial 10 days of warfarin therapy. Biomark Med. 2019;13(18):1599–1607. doi:10.2217/bmm-2019-0033
43. Kang M, Yu J, Sung HH, et al. Prognostic impact of the pretreatment aspartate transaminase/alanine transaminase ratio in patients treated with first-line systemic tyrosine kinase inhibitor therapy for metastatic renal cell carcinoma. Int J Urol. 2018;25(6):596–603. doi:10.1111/iju.13574
44. Miyake H, Matsushita Y, Watanabe H, et al. Significance of De Ritis (Aspartate Transaminase/Alanine Transaminase) ratio as a significant prognostic but not predictive biomarker in Japanese patients with metastatic castration-resistant prostate cancer treated with cabazitaxel. Anticancer Res. 2018;38(7):4179–4185. doi:10.21873/anticanres.12711
45. Lee H, Lee SE, Byun SS, Kim HH, Kwak C, Hong SK. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localized renal cell carcinoma: a propensity score-matched study. BJU Int. 2017;119(2):261–267. doi:10.1111/bju.13545
46. Cho YH, Hwang JE, Chung HS, et al. The De Ritis (aspartate transaminase/alanine transaminase) ratio as a predictor of oncological outcomes in patients after surgery for upper urinary tract urothelial carcinoma. Int Urol Nephrol. 2017;49(8):1383–1390. doi:10.1007/s11255-017-1613-z
47. Rief P, Pichler M, Raggam R, et al. The AST/ALT (De-Ritis) ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine. 2016;95(24):e3843. doi:10.1097/md.0000000000003843
48. Opio CK, Seremba E, Ocama P, Lalitha R, Kagimu M, Lee WM. Diagnosis of alcohol misuse and alcoholic liver disease among patients in the medical emergency admission service of a large urban hospital in Sub-Saharan Africa; a cross sectional study. Pan Afr Med J. 2013;15:23. doi:10.11604/pamj.2013.15.23.2040
49. Torkadi PP, Apte IC, Bhute AK. Biochemical evaluation of patients of alcoholic liver disease and non-alcoholic liver disease. Indian J Clin Biochem. 2014;29(1):79–83. doi:10.1007/s12291-013-0310-7
50. Tan X, Xiao K, Liu W, Chang S, Zhang T, Tang H. Prognostic factors of distal cholangiocarcinoma after curative surgery: a series of 84 cases. Hepatogastroenterology. 2013;60(128):1892–1895.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



