Back to Journals » OncoTargets and Therapy » Volume 12
A Feedback Loop Regulation Of LINC01433 And YAP Promotes Malignant Behavior In Gastric Cancer Cells
Authors Zhang C, Qian H, Liu K, Zhao W, Wang L
Received 12 July 2019
Accepted for publication 1 September 2019
Published 1 October 2019 Volume 2019:12 Pages 7949—7962
DOI https://doi.org/10.2147/OTT.S222903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Cao Zhang,* Haiquan Qian,* Ke Liu, Wei Zhao, Lei Wang
The Department of Gastrointestinal Surgery, General Hospital of Ningxia Medical University, Yinchuan City, The Ningxia Hui Autonomous Region, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Wang
The Department of Gastrointestinal surgery, General Hospital of Ningxia Medical University, No.804, Shengli South Road, Yinchuan City, The Ningxia Hui Autonomous Region 750004, People’s Republic of China
Email [email protected]
Background: Gastric cancer (GC) is one of the most common cancers and the second leading cause of cancer-related death worldwide. Long noncoding RNAs (lncRNAs) are associated with GC development and progression. However, the functional roles and underlying mechanism of LINC01433 on GC progression remain elusive.
Methods: Firstly, the expression of LINC01433 was examined in 76 pairs of primary GC and corresponding adjacent non-tumorous tissues. Next, overexpression and knockdown experiments were conducted in GC cells to explore the effect of LINC01433 on the malignant behaviors of GC cells. Then, the interaction between LINC01433 and YAP was detected by RNA immunoprecipitation (RIP) and RNA pull-down assays.
Results: We found that LINC01433 was significantly upregulated in GC tissues and cell lines and correlated with poor prognosis. Through gain- and loss-of-function experiments, we demonstrated that LINC01433 promoted proliferation, migration, invasion and chemotherapy resistance in GC cells. Further mechanistic investigation revealed that LINC01433 could stabilize oncoprotein YAP through enhancing the interaction between deubiquitinase USP9X and YAP. LINC01433 decreased the phosphorylation of YAP via suppressing YAP-LATS1 association. Intriguingly, YAP directly bound to LINC01433 promoter region and activated its transcription. Thus, LINC01433 and YAP formed a positive feedback loop.
Conclusion: Collectively, our study demonstrates that the positive feedback loop between LINC01433 and YAP promotes GC progression, and implies that the LINC01433-YAP feedback loop may be a promising therapeutic target for GC treatment.
Keywords: protein stability, USP9X, YAP, LATS1
Introduction
Despite an increase in its public awareness, gastric cancer (GC) remains one of the most common cancers and the second leading cause of cancer-related death, and it mainly occurs in Eastern Asia.1 The treatments for GC include surgical resection, chemotherapy and radiotherapy.2 However, most GC patients were diagnosed at advanced stage and usually had poor overall survival time due to GC recurrence and metastasis.3 Some genes involved in cell growth, metastasis, chemoresistance and angiogenesis have been proved to contribute to GC progression,4–6 but the underlying mechanisms have not been fully elucidated. Therefore, it is necessary to explore the molecular mechanism and identification of novel therapeutic targets for GC.
Noncoding RNAs longer than 200 nucleotides are defined as long noncoding RNAs (lncRNAs). Emerging evidence has demonstrated that lncRNAs are closely associated with human diseases and participate in tumor growth, metastasis, differentiation, angiogenesis and drug resistance.7,8 With great development of high-throughput RNA sequencing technology, increasing numbers of dysregulated lncRNAs in GC have been identified. For example, HOTAIR is elevated in GC tissues and cells and promotes GC metastasis by binding to polycomb repressive complex 2 (PRC2) and epigenetically silencing miR-34a expression.9 PVT1 is upregulated and significantly associated with high-microvessel density and poor prognosis in GC. PVT1 directly interacts with the signal transducer activator phospho-STAT3 and increases its protein stability by protecting it from proteasome-dependent degradation, and then induces angiogenesis within tumors and tumor growth.10 lncR-D63785 acts as a competing endogenous RNA (ceRNA) of miR-422a to promote chemoresistance by blocking miR-422-mediated MEF2D suppression in GC.11 However, the exact biological functions of most lncRNAs in GC development and progression remain elusive.
The Hippo pathway is a critical signaling pathway that regulates cell growth, proliferation, differentiation and tissue homeostasis.12,13 The central axis of Hippo pathway comprises a phosphorylation cascade of MST1/2-LATS1/2-YAP/TAZ. When the upstream Hippo signaling pathway is activated, LATS1/2 can be phosphorylated by MST1/2 kinases. Then, LATS1/2 phosphorylates YAP and TAZ and increases 14-3-3 binding to phosphorylated YAP/TAZ, leading to the accumulation of the YAP/TAZ in the cytoplasm.14 Conversely, dephosphorylated YAP1/TAZ translocates the nucleus and promotes the transcription of genes regulating proliferation, differentiation and migration through binding to the TEAD1-4 transcription factors. Elevated YAP expression and nuclear accumulation are widespread in cancers and associated with growth, metastasis and drug resistance of GC.15,16 Recently, it was found that some lncRNAs participate in regulating YAP. For example, lncRNA B4GALT1-AS1 promotes osteosarcoma cells proliferation, migration, stemness and chemotherapeutic resistance through recruiting HuR to enhance YAP mRNA stability.17 lncRNA uc.134 represses hepatocellular carcinoma progression by inhibiting the CUL4A-mediated ubiquitination of LATS1 and increasing YAP phosphorylation.18 lncARSR facilitates the self-renewal, tumorigenicity and metastasis of renal tumour-initiating cells through suppressing LATS1-induced YAP phosphorylation and promoting YAP nuclear translocation.19 However, the regulatory mechanism of YAP by lncRNAs in GC progression has not been fully understood.
LINC01433 is a newly identified lncRNA, which locates in chromosome 20p13 and contains 840 nucleotides. Recent studies reported that LINC01433 is overexpressed in non-small cell lung cancer, hepatocellular carcinoma and breast cancer, and acts as an oncogene to modulate proliferation, migration and invasion of cancer cells.20–22 However, the functional significance and underlying mechanisms of LINC01433 in GC progression are poorly understood. Here, our results revealed that LINC01433 promotes cell proliferation, migration, invasion and chemoresistance through stabilizing YAP and enhancing its activation, which highlights the possible development of novel therapeutic strategies for GC.
Materials And Methods
Tissues Collection
Seventy-six pairs of primary GC and corresponding adjacent non-tumorous tissues from patients diagnosed with GC and underwent surgery at the General Hospital of Ningxia Medical University. None of these patients received chemotherapy or radiotherapy. The tissue samples were immediately frozen in liquid nitrogen and stored at −80°C until use for further detection. Written informed consent was obtained from all patients, and the study protocol was approved by the ethics committee of General Hospital of Ningxia Medical University. This work was conducted in accordance with the Declaration of Helsinki.
Cell Lines
Normal gastric epithelial cells (GES-1), human GC cell lines (AGS, MGC803, BGC823, HGC27, SGC7901 and MKN45) cells and HEK293T cells were purchased from the Cell Center of Shanghai Institutes for Biological Sciences and cultured in RPMI-1640 medium (Gibco, USA) or DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibico, USA), 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C with 5% CO2 in a humidified atmosphere.
Cell Proliferation/viability Detection
Cell proliferation or viability was measured via CCK-8 assay. 2 × 103 indicated cells were seeded into 96-well plates at 37°C with 5% CO2. At indicated time point, 10 μL Cell Counting Kit-8 (CCK-8, Dojindo, Beijing, China) was added into each well and incubated with cells for 1.5 hrs. Then, the absorbance (OD 450 nm) was measured using the Universal Microplate Spectrophotometer (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Cell Migration And Invasion Assay
For migration assay, cells were suspended in 200 μL serum-free RPMI-1640 and seeded into a 24-well Boyden chamber (8 μm pore size, Corning, USA). For invasion assay, cells were suspended in 200 μL serum-free RPMI-1640 and seeded into a 24-well Boyden chamber (8 μm pore size, Corning, USA) with Matrigel-pre-coated inserts (BD, USA). 500 μL DMEM medium with 10% FBS was added into the down chambers. After incubation for 24 hrs, cells attached to the chambers’ lower surface were fixed with 4% paraformaldehyde, and then stained with 0.1% crystal violet, and counted under a microscope.
Constructs And Viruses
shRNA targeting LINC01433 or YAP or USP9X was inserted into pLKO.1 plasmid. The target sequence is listed as follows: LINC01433 shRNA-1 (sh1): GTGCAAACCTCAGAACTGT; LINC01433 shRNA-2 (sh2): TCCTCACTGTCATGCACTA; YAP shRNA (shYAP): GGCAAAGACATCTTCTGGT; USP9X shRNA (shUSP9X): GAGAGTTTATTCACTGTCTTA. Full-length LINC01433 was cloned into pLV-puro plasmid. The lentiviral supernatant was generated by transfecting HEK293T cells with psPAX2 and pMD.2G and collected 48 hrs later. Supernatants were passed through a 0.45-μm filter and used to infect the cells with the addition of 8 μg/mL polybrene (Sigma-Aldrich). Stable cells were selected by using 3 μg/mL puromycin.
RNA Extraction And Quantitative Real-Time PCR (qRT-PCR)
RNA isolation was performed by using Trizol reagent (Invitrogen) according to the standard protocol. cDNA was synthesized by using the Script Reverse Transcription Supermix Kit (Bio-Rad) according to the manufacturer’s instructions. qRT-PCR analysis was performed by SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) and the ABI PRISM 7900 Sequence Detection System (Applied Biosystems). The relative expression was calculated by the 2−ΔΔCt method and normalized to that of GAPDH. The primer sequences for indicated genes were listed as follows: LINC01433-F: TGCCTTTGCTGCTGTATGA, LINC01433-R: CTCCAAAGGACAGGCATGAA; YAP-F: CAGACAGTGGACTAAGCATGAG, YAP-R: CAGGGTGCTTTGGTTGATAGTA; GAPDH-F: GGCAAAGACATCTTCTGGT, GAPDH-R: CAGGCAATGCGGAATATCA; CTGF-F: GCCCAGACCCAACTATGATTAG, CTGF-R: GGAGGCGTTGTCATTGGTAA; CYR61-F: GGGTCTGTGACGAGGATAGTA, CYR61-R: CATCGAATCCCAGCTCCTTAC; MYC-F: CCTCAACGTTAGCTTCACCA, MYC-R: CTCCTCGTCGCAGTAGAAATAC.
Western Blot
Total protein samples were extracted by RIPA lysis buffer (Beyotime, Beijing, China) and separated on SDS-PAGE, and then transferred to PVDF membranes (Millipore, USA). After block with 5% nonfat milk, the membranes were incubated with primary antibodies overnight at 4°C, including YAP (Cell Signaling), p-YAP (Cell Signaling), LATS1 (Cell Signaling), USP9X (Cell Signaling) or GAPDH (Cell Signaling). After wash, the membranes were incubated with a secondary antibody. Protein bands were observed using Western Chemiluminescent HRP Substrate (Millipore).
Cell Apoptosis Detection
Indicated cells were harvested by trypsinization. The cells were stained with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide, and then analyzed by flow cytometry (FACScan; BD Biosciences).
Cell Cycle Detection
Indicated cells were fixed by 70% ethanol overnight at 4°C, stained with propidium iodide and analyzed by flow cytometry (FACScan; BD Biosciences).
RNA Immunoprecipitation (RIP) And RNA Pull-Down Assays
RIP assay was performed by EZ-Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore) according to the manufacturer’s instructions. RNA pull-down assay was performed as previously described.23
Chromatin Immunoprecipitation Assay (ChIP)
Chromatin immunoprecipitation (ChIP) experiments were performed using the Magna ChIP Kit (Millipore) according to the manufacturer’s instructions as previously described.24
Co-Immunoprecipitation Assay
Cells were collected and lysed with an IP lysis buffer (Beyotime Institute of Biotechnology). Total protein was incubated with Protein G-agarose suspension (Millipore) for 3 hrs at 4°C on a rocking platform to reduce non-specific binding. After removing the beads, the supernatant was supplemented with the primary antibodies followed by incubation for an additional 3 hrs at 4°C. Protein G-agarose was then added to each immunoprecipitation mixture, and the incubation was continued overnight at 4°C on a rocking platform. The immunoprecipitates were collected by centrifugation and washed three times. After the loading buffer was added, the agarose was boiled and subjected to Western blot analysis.
Luciferase Reporter Assay
Cells were plated in 6-well plates at a density of 2 ×105 per well. Cells were transfected with 0.3 µg of promoter-luciferase plasmid along with 1 μg of pLKO.1-shYAP or pcDNA3-YAP in each well. To normalize transfection efficiency, cells were also co-transfected with 0.1 µg of the pRL-CMV (Renilla luciferase). 48 hrs later, luciferase activity was measured using the Dual-Luciferase Assay kit (Promega) according to the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed using SPSS 20.0 software (IBM, SPSS, Chicago, IL, USA). The correlation between LINC01433 and clinical features was analyzed by Chi-square tests. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnett’s test among multiple groups. The Student t-test was used to analyze the differences between two groups. Survival curves were calculated and analyzed by using the Kaplan–Meier method and log-rank test. A P value < 0.05 was considered statistically significant.
Results
LINC01433 Is Upregulated In GC Cells And Tissues And Correlated With GC Progression
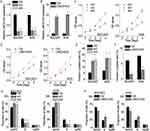
To investigate differential expression of LINC01433 in GC, 76 patients with GC were evaluated. The expression of LINC01433 in GC and corresponding normal tissues was measured by qRT-PCR. It was observed that LINC01433 expression levels in GC tissues were significantly higher than those in normal tissues (Figure 1A). To determine whether LINC01433 expression levels are closely associated with the GC progression, we analyzed the relationship between LINC01433 expression and clinicopathological features of these GC patients. Patients were divided into two groups according to the median of LINC01433 expression in GC tissues. Statistical analysis represented a significant correlation between LINC01433 expression and tumor invasion, tumor size, TNM stage and lymph node metastasis (Table 1). However, LINC01433 expression was not associated with other factors including age, gender and differentiation grade in GC. Moreover, Kaplan–Meier survival analysis was used to compare overall survival rates of GC patients with different levels of LINC01433. The results showed that the GC patients with high LINC01433 expression had poorer prognosis than low-level LINC01433 group (Figure 1B). In addition, the expression level of LINC01433 in normal gastric epithelial cells (GES-1) and six GC cell lines (AGS, MGC803, BGC823, HGC27, SGC7901 and MKN45) were determined using qRT-PCR. As shown in Figure 1C, the LINC01433 expression was much higher in all GC cell lines than GES-1 cells.
 |
Table 1 The Correlation Between LINC01433 Expression And Clinicopathological Features Of Gastric Cancer Patients |
LINC01433 Facilitates Cell Proliferation, Suppresses Cell Apoptosis And Triggers Cell Cycle Progression
We then investigate the functional roles of LINC01433 expression in the progression of GC. The expression levels of LINC01433 were not significantly different among these cell lines (Figure 1C). Considering the transfection efficacy, BGC823 and AGS cells were selected for further experiments. LINC01433 expression was silenced in BGC823 and AGS cells by transfection with two different lentiviral particles expressing LINC01433 shRNA (sh1 and sh2) and was overexpressed by transfection with lentiviral particles expressing full-length LINC01433 (Figure 2A and B). The effect of LINC01433 expression on cell proliferation was assessed using a CCK-8 kit. The results demonstrated that knockdown endogenous LINC01433 significantly reduced the proliferative ability of both BGC823 and AGS cells (Figure 2C), whereas LINC01433 overexpression promoted BGC823 and AGS cell proliferation (Figure 2D).
To gain insights into the mechanism by which LINC01433 enhances GC cell proliferation, we analyzed differences in cell apoptosis and cell cycle distributions. Fluorescence-activated cell sorting (FACS) analysis and AnnexinV/PI staining were used to measure cell apoptosis. The results showed that LINC01433-knockdown BGC823 and AGS cells had a significantly higher percentage of Annexin V-positive cells than control cells did (Figure 2E), while overexpression of LINC01433 inhibited cellular apoptosis (Figure 2F). Moreover, the results of cell cycle assays by FACS analysis indicated that knockdown of LINC01433 expression significantly elevated the proportion of cells in G0/G1 phase and reduced the proportion of cells in S phase (Figure 2G). In contrast, LINC01433 overexpression triggered cell cycle progression beyond the G1/S transition in BGC823 and AGS cells (Figure 2H).
LINC01433 Promotes GC Cell Migration And Invasion
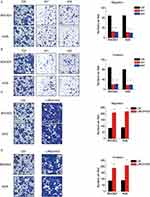
The correlation of LINC01433 expression and tumor invasion and lymph node metastasis suggested a promotion of LINC01433 in GC progression. To validate this hypothesis, we examined the effect of LINC01433 knockdown and overexpression on the migration and invasive behavior of BGC823 and AGS cells. The results of transwell assay showed that the migration and invasive ability of the BGC823 and AGS cells were dramatically impaired by depletion of endogenous LINC01433 (Figure 3A and B). Conversely, LINC01433 overexpression enhanced migration and invasion of BGC823 and AGS cells (Figure 3C and D).
LINC01433 Reduces The Sensitivity Of GC Cells To Chemotherapy
We then tested whether LINC01433 affects GC cells response to chemotherapy. The responses to doxorubicin (DOX) and cisplatin were measured. The IC50 value of both DOX and cisplatin on BGC823 and AGS cells was significantly decreased after LINC01433 knockdown (Figure 4A). Moreover, to test the effect of LINC01433 on the chemotherapy-induced apoptosis, apoptosis assays by flow cytometry were utilized. The results showed that LINC01433 knockdown led to a significant increased susceptibility of BGC823 and AGS cells to DOX or cisplatin-induced cytotoxicity (Figure 4B). Conversely, overexpression LINC01433 exerts opposite effect (Figure 4C and D). These results demonstrate an important role of LINC01433 in regulating chemotherapeutic response.
LINC01433 Stabilizes YAP Through USP9X
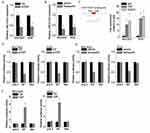
Next, we explored the molecular mechanism underlying LINC01433-mediated phenotypes of GC cells. LncRNAs regulate their target genes by interacting with RNA-binding proteins or acting as a ceRNA. We first analyzed the distribution of LINC01433 in GC cells using subcellular fractionation analyses. The results showed that LINC01433 was distributed in both the cytoplasm and nucleus, but the ratio of LINC01433 in the nucleus was higher (Figure 5A). We performed RNA pull-down assay and followed by mass spectrometry to identify proteins associated with LINC01433. YAP, a core effector of the Hippo pathway, was initially identified as a protein that was present in LINC01433-associated protein samples. Given that elevated YAP expression is widespread in human cancers and associated with growth, metastasis and drug resistance of GC,15,16 we chose YAP as the research object of the mechanism. To validate the interaction of LINC01433 with YAP, RNA pull-down assay was performed. A positive signal was observed in proteins pulled down with LINC01433 RNA but not in samples bound with negative control antisense LINC01433 (Figure 5B). We performed a RIP assay for further confirmation, in which the RNA-YAP complex was immunoprecipitated using an anti-YAP antibody. LINC01433 could be significantly enriched by anti-YAP antibodies compared with the immunoglobulin G (IgG) (Figure 5C). Furthermore, using a series of deletion-mapping analyses, we identified a 230 nt region in the 5ʹ terminal region of the LINC01433 transcript, which is essential for the LINC01433-YAP interaction (Figure 5D). Collectively, these data demonstrate YAP as a LINC01433-associated protein.
We then determined the regulatory relationship of LINC01433-YAP interaction. Knockdown of LINC01433 significantly decreased the protein level of YAP but did not change the mRNA level of YAP (Figure 5E and F), indicating that LINC01433 stabilized YAP protein. To further explore the mechanism of LINC01433-mediated YAP upregulation, we examined if LINC01433 affected the half-life of YAP using cycloheximide (CHX) (protein synthesis inhibitor). It was observed that knockdown of LINC01433 dramatically accelerated the degradation of YAP in BGC823 cells (Figure 5G), while overexpression of LINC01433 significantly potentiated YAP protein stability in AGS cells (Figure 5H). Moreover, LINC01433 knockdown increased YAP ubiquitination level (Figure 5I), whereas ectopic expression of LINC01433 significantly attenuated the level of YAP ubiquitination (Figure 5J). When the proteasome inhibitor MG132 was added into the culture medium, the YAP protein expression in LINC01433-silencing cells was significantly increased and reached a level that was comparable to that in control cells (Figure 5K), indicating that LINC01433 depletion attenuated the proteasome-mediated degradation of YAP.
Deubiquitinating enzymes are responsible for maintaining protein stability. Given that LINC01433 stabilized YAP protein, we speculated that deubiquitinating enzymes were involved in this process. A previous study demonstrated that deubiquitinase USP9X could interact with and deubiquitinate YAP, and then enhanced its protein stability.25 Interestingly, the results of RNA pull-down followed by mass spectrometry analysis demonstrated that USP9X potentially associated with LINC01433. To validate this, RIP assay was performed and showed that LINC01433 could be significantly pulled down by anti-USP9X antibodies compared to the negative control IgG (Figure 6A). To detect whether LINC01433 stabilized YAP stability through USP9X, we knocked down the endogenous USP9X expression in LINC01433-overexpressed BGC823 and AGS cells through transfection of shRNA targeting USP9X. We found that depletion of USP9X expression almost abolished the YAP expression increased by LINC01433 overexpression (Figure 6B). To assess whether LINC01433 could influence the interaction between USP9X and YAP, the co-immunoprecipitation assay was performed. It was observed that knockdown of LINC01433 significantly attenuated the interaction between USP9X and YAP in BGC823 cells (Figure 6C). These results suggest that LINC01433 stabilizes YAP protein through enhancing the USP9X-YAP interaction.
LINC01433 Suppresses The Phosphorylation Of YAP
It has been reported that lncRNA could regulate the phosphorylation of its interacting protein.26,27 We then determined whether LINC01433 had an effect on the phosphorylation of YAP. We found that knockdown of LINC01433 increased the phosphorylation of YAP (Figure 7A), while overexpression reduced YAP phosphorylation (Figure 7B). LATS1 directly phosphorylates YAP, resulting in cytoplasmic sequestration. Interestingly, the result of co-immunoprecipitation assays showed that the interaction between LATS1 and YAP was enhanced by LINC01433 knockdown in BGC823 cells (Figure 7C). Conversely, LINC01433 overexpression attenuated the interaction between LATS1 and YAP in AGS cells (Figure 7D). Additionally, immunofluorescence assay showed that knockdown of LINC01433 suppressed the translocation of YAP into the nucleus, while ectopic expression of LINC01433 led to nuclear translocation of YAP (Figure S1).
Decreased phosphorylation of YAP translocated from cytoplasm into nucleus and led to the expression of downstream oncoproteins through interaction with transcriptional factors TEADs, such as connective tissue growth factor (CTGF), cysteine-rich angiogenic inducer 61 (CYR61) and MYC.28 We detected these genes expression following LINC01433 alteration. The results of qRT-PCR showed the expression of CTGF, CYR61 and MYC was significantly decreased by LINC01433 knockdown (Figure 7E), whereas overexpression of LINC01433 induced the transcription of CTGF, CYR61 and MYC (Figure 7F).
LINC01433 Is Transcriptionally Activated By YAP
Finally, we hypothesized that whether LINC01433 could form feedback loop with YAP. BGC823 and AGS cells were transfected with shRNAs against YAP. After 48 hrs, the LINC01433 expression was detected by qRT-PCR. As shown in Figure 8A, depletion of YAP dramatically decreased the LINC01433 expression. Additionally, the treatment of YAP inhibitor verteporfin led to a significant decrease of LINC01433 expression in both BGC823 and AGS cells (Figure 8B).
Since YAP exerts its transcriptional coactivator function predominantly via interaction with transcription factor TEAD1,29 we used the JASPAR database to analyze the promoter region of LINC01433 gene for TEAD1-YAP-binding sites. Notably, one TEAD1-YAP-binding site was identified in the promoter region of LINC01433 (Figure 8C). To validate the binding of YAP on LINC01433 promoter, a ChIP assay was performed, and the result demonstrated that YAP and TEAD1 could bind to LINC01433 promoter at this site (Figure 8C). To further confirm the competency for YAP binding, a luciferase reporter assay was conducted. Both transfection of YAP shRNA and verteporfin treatment reduced the luciferase activity of LINC01433 promoter, while mutation of the TEAD1-YAP-binding site abrogated this effect (Figure 8D and E). Conversely, overexpression of YAP increased the luciferase activity of wild-type LINC01433 promoter but fails to stimulate the mutant site in the promoter region of LINC01433 (Figure 8F). These results suggest that YAP activates LINC01433 transcription and forms a positive feedback loop regulation with LINC01433.
Discussion
lncRNAs play important roles in various types of human cancers, including GC, which is one of the most common malignancies in China.30 Increasing research demonstrates that some lncRNAs may function as novel diagnostic markers or prognostic predictor for GC patients.5,8 Herein, we found that the expression level of LINC01433 was significantly increased in GC tissues and cell lines. Upregulation of LINC01433 also associated tumor invasion, tumor size, TNM stage and lymph node metastasis, and predicted poor prognosis of patients with GC. Loss- and gain-of-function experiments demonstrated that LINC01433 promoted GC cell proliferation, migrationand invasion and suppressed the sensitivity of GC to chemotherapy. Our findings reveal a novel functional lncRNA regulating GC progression.
We identified that LINC01433 could interact with the core effector of the Hippo pathway, YAP. Previous studies have suggested that some lncRNAs can regulate the stability of its interacting protein. For example, FAL1 associates with the epigenetic repressor BMI1 and enhances its stability in order to regulate the transcription of a number of genes including CDKN1A.31 LUCAT1 regulates the stability of DNMT1 and inhibits the expression of tumor suppressors through DNA methylation, enhancing growth and metastasis of esophageal squamous cell carcinoma.32 In addition, lncRNA UCA1 regulates GRK2 protein stability by promoting Cbl-c-mediated GRK2 ubiquitination and degradation, which increases the metastatic ability of GC cells.33 Here, our data demonstrated that LINC01433 promoted the interaction between YAP and deubiquitinase USP9X, thereby inducing the stabilization of YAP protein. In combination with these findings, the functional LINC01433-YAP interaction that we characterized in this study further emphasizes the importance of lncRNAs in the regulation of protein ubiquitination modification.
Phosphorylation modification of protein could influence its activation, subcellular location and stability.34,35 Recently, some lncRNAs have been found to influence the phosphorylation of their interacting proteins. For example, LINC00675 interacts with vimentin and enhanced its phosphorylation level on Ser83 to result in the collapse of vimentin filament, leading to GC cell metastasis inhibition.27 LncRNA HULC specifically binds to Y-box binding protein 1 (YB-1) protein, a major component of translationally inactive messenger ribonucleoprotein particles, and promotes YB-1 phosphorylation, which leads to the release of YB-1 from its bound mRNA.26 Here, we demonstrated that LINC01433 suppressed YAP phosphorylation and activated the transcription of its downstream oncoproteins, such as CTGF, CYR61 and MYC. Mechanistically, LINC01433 suppressed the interaction between LATS1 and YAP, indicating that LINC01433 may competitively bind YAP.
As a transcription factor, YAP carries out its functions by binding to the promoter of downstream effector genes and stimulating their transcriptional activities. However, to date, no lncRNAs have been reported to be the targets of YAP. We found that YAP bound to the promoter region of LINC01433 to activate its transcription, suggesting that LINC01433 formed a positive feedback loop with YAP. Previous study also reported some similar regulatory manner. For instance, lncRNA PVT1 directly bind FOXM1 protein and increased its stability. FOXM1 directly binds to the PVT1 promoter to activate its transcription.36 In addition, LINC01296 upregulates Twist1 expression through sponging miR-598, and Twist1 can bind with the promoter of LINC01296 and thus activates its transcriptional level.37 These results along with ours might reflect a reciprocal regulatory mechanism between transcriptional factors and lncRNAs, which consequently amplifies their mutual oncogenic functions in cancers.
In summary, our study identified a novel positive feedback regulation between LINC01433 and YAP, which contributes to the GC development and progression. Our results also suggest that the LINC01433 expression may be a useful biomarker for prognosis and targeting the LINC01433-YAP loop might be a potential strategy for GC treatment.
Acknowledgments
This work was supported by Ningxia Natural Science Foundation (No. 2019AAC03200).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626. doi:10.1177/1010428317714626
3. Peng L, Yu K, Li Y, Xiao W. Gastric metastasis of recurrent hepatocellular carcinoma: a case report and literature review. J Cancer Res Ther. 2018;14(Supplement):S1230–S1232. doi:10.4103/0973-1482.199379
4. Fu Y, Du P, Zhao J, Hu C, Qin Y, Huang G. Gastric cancer stem cells: mechanisms and therapeutic approaches. Yonsei Med J. 2018;59(10):1150–1158. doi:10.3349/ymj.2018.59.10.1150
5. Lin MT, Song HJ, Ding XY. Long non-coding RNAs involved in metastasis of gastric cancer. World J Gastroenterol. 2018;24(33):3724–3737. doi:10.3748/wjg.v24.i33.3724
6. Ng WL, Mohd Mohidin TB, Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol. 2018;15(8):995–1005. doi:10.1080/15476286.2018.1486659
7. Hahne JC, Valeri N. Non-coding RNAs and resistance to anticancer drugs in gastrointestinal tumors. Front Oncol. 2018;8:226.
8. Fanelli GN, Gasparini P, Coati I, et al. long-noncoding RNAs in gastroesophageal cancers. Non-coding RNA Res. 2018;3(4):195–212. doi:10.1016/j.ncrna.2018.10.001
9. Liu YW, Sun M, Xia R, et al. LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial-to-mesenchymal transition in human gastric cancer. Cell Death Dis. 2015;6:e1802. doi:10.1038/cddis.2015.150
10. Zhao J, Du P, Cui P, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37(30):4094–4109. doi:10.1038/s41388-018-0250-z
11. Zhou Z, Lin Z, He Y, et al. The long noncoding RNA D63785 regulates chemotherapy sensitivity in human gastric cancer by targeting miR-422a. Mol Ther Nucleic Acids. 2018;12:405–419. doi:10.1016/j.omtn.2018.05.024
12. Xie H, Wu L, Deng Z, Huo Y, Cheng Y. Emerging roles of YAP/TAZ in lung physiology and diseases. Life Sci. 2018;214:176–183. doi:10.1016/j.lfs.2018.10.062
13. Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi:10.1016/j.devcel.2010.09.011
14. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24(1):72–85. doi:10.1101/gad.1843810
15. Pan Z, Tian Y, Zhang B, et al. YAP signaling in gastric cancer-derived mesenchymal stem cells is critical for its promoting role in cancer progression. Int J Oncol. 2017;51(4):1055–1066. doi:10.3892/ijo.2017.4101
16. Lu T, Sun L, Zhu X. Yes-associated protein enhances proliferation and attenuates sensitivity to cisplatin in human gastric cancer cells. Biomed Pharmacother. 2018;105:1269–1275. doi:10.1016/j.biopha.2018.06.031
17. Li Z, Wang Y, Hu R, Xu R, Xu W. LncRNA B4GALT1-AS1 recruits HuR to promote osteosarcoma cells stemness and migration via enhancing YAP transcriptional activity. Cell Prolif. 2018;51(6):e12504. doi:10.1111/cpr.2018.51.issue-6
18. Ni W, Zhang Y, Zhan Z, et al. A novel lncRNA uc.134 represses hepatocellular carcinoma progression by inhibiting CUL4A-mediated ubiquitination of LATS1. J Hematol Oncol. 2017;10(1):91. doi:10.1186/s13045-017-0449-4
19. Qu L, Wu Z, Li Y, et al. A feed-forward loop between lncARSR and YAP activity promotes expansion of renal tumour-initiating cells. Nat Commun. 2016;7:12692. doi:10.1038/ncomms12692
20. Qian B, Wang X, Mao C, et al. Long non-coding RNA linc01433 promotes migration and invasion in non-small cell lung cancer. Thoracic Cancer. 2018;9(5):589–597. doi:10.1111/1759-7714.12623
21. Huang H, Bu YZ, Zhang XY, Liu J, Zhu LY, Fang Y. LINC01433 promotes hepatocellular carcinoma progression via modulating the miR-1301/STAT3 axis. J Cell Physiol. 2019;234(5):6116–6124. doi:10.1002/jcp.27366
22. Wu M, Wu W, Ding J, Yang J. LINC01433/miR-2116-3p/MYC feedback loop promotes cell proliferation, migration, and the epithelial-mesenchymal transition in breast cancer. Cancer Biother Radiopharm. 2019. doi:10.1089/cbr.2019.2772
23. Yang F, Zhang L, Huo XS, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54(5):1679–1689. doi:10.1002/hep.24563
24. Xu TP, Ma P, Wang WY, et al. KLF5 and MYC modulated LINC00346 contributes to gastric cancer progression through acting as a competing endogeous RNA and indicates poor outcome. Cell Death Differ. 2019. doi:10.1038/s41418-018-0236-y
25. Li L, Liu T, Li Y, et al. The deubiquitinase USP9X promotes tumor cell survival and confers chemoresistance through YAP1 stabilization. Oncogene. 2018;37(18):2422–2431. doi:10.1038/s41388-018-0134-2
26. Li D, Liu X, Zhou J, et al. Long noncoding RNA HULC modulates the phosphorylation of YB-1 through serving as a scaffold of extracellular signal-regulated kinase and YB-1 to enhance hepatocarcinogenesis. Hepatology. 2017;65(5):1612–1627.
27. Zeng S, Xie X, Xiao YF, et al. Long noncoding RNA LINC00675 enhances phosphorylation of vimentin on Ser83 to suppress gastric cancer progression. Cancer Lett. 2018;412:179–187. doi:10.1016/j.canlet.2017.10.026
28. Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. The TEAD family and its oncogenic role in promoting tumorigenesis. Int J Mol Sci. 2016;17:1.
29. Stein C, Bardet AF, Roma G, et al. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 2015;11(8):e1005465. doi:10.1371/journal.pgen.1005465
30. Aoyama T, Yoshikawa T. Adjuvant therapy for locally advanced gastric cancer. Surg Today. 2017;47(11):1295–1302. doi:10.1007/s00595-017-1493-y
31. Hu X, Feng Y, Zhang D, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26(3):344–357. doi:10.1016/j.ccr.2014.07.009
32. Yoon JH, You BH, Park CH, Kim YJ, Nam JW, Lee SK. The long noncoding RNA LUCAT1 promotes tumorigenesis by controlling ubiquitination and stability of DNA methyltransferase 1 in esophageal squamous cell carcinoma. Cancer Lett. 2018;417:47–57. doi:10.1016/j.canlet.2017.12.016
33. Wang ZQ, He CY, Hu L, et al. Long noncoding RNA UCA1 promotes tumour metastasis by inducing GRK2 degradation in gastric cancer. Cancer Lett. 2017;408:10–21. doi:10.1016/j.canlet.2017.08.013
34. Yang Q, Langston JC, Tang Y, Kiani MF, Kilpatrick LE. The role of tyrosine phosphorylation of protein kinase C delta in infection and inflammation. Int J Mol Sci. 2019;20:6.
35. Montagnani V, Stecca B. Role of protein kinases in hedgehog pathway control and implications for cancer therapy. Cancers. 2019;11:4. doi:10.3390/cancers11040449
36. Xu MD, Wang Y, Weng W, et al. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin Cancer Res. 2017;23(8):2071–2080. doi:10.1158/1078-0432.CCR-16-0742
37. Xu L, Wei B, Hui H, et al. Positive feedback loop of lncRNA LINC01296/miR-598/Twist1 promotes non-small cell lung cancer tumorigenesis. J Cell Physiol. 2019;234(4):4563–4571. doi:10.1002/jcp.27235
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.