Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
A Cross-Sectional Study of the Correlation Between the Atherogenic Index of Plasma and Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes
Received 24 May 2022
Accepted for publication 18 July 2022
Published 30 July 2022 Volume 2022:15 Pages 2227—2234
DOI https://doi.org/10.2147/DMSO.S375300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Jie Lin,1– 4 Hang Li,1– 4 Qin Wan1– 4
1Department of Endocrinology and Metabolism, The Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 2Metabolic Vascular Disease Key Laboratory of Sichuan Province, Luzhou, People’s Republic of China; 3Sichuan Clinical Research Center for Nephropathy, Luzhou, People’s Republic of China; 4Cardiovascular and Metabolic Diseases Key Laboratory of Luzhou, Luzhou, People’s Republic of China
Correspondence: Qin Wan, Tel +86 138 8274 6971, Email [email protected]
Abstract:
Purpose: The main objective of this study was to examine the possible association between the atherogenic index of plasma (AIP) and the prevalence of nonalcoholic fatty liver disease (NAFLD) in Chinese individuals with type 2 diabetes mellitus (T2DM).
Patients and methods: In this survey, data from 1074 patients with T2DM were retrospectively extracted. The correlations between each variable and NAFLD were determined by univariate analysis, and then, the statistically significant variables were evaluated for their association with AIP and NAFLD by multivariate regression analysis.
Results: AIP levels were significantly higher in all males and females with NAFLD than those without NAFLD (p< 0.001). The prevalence of NAFLD increased progressively throughout the AIP quartiles (trend P < 0.001) and accounted for possible variables in Model 3 of the multivariate logistic regression analysis (OR: 2244.984). In terms of sensitivity and specificity, the AIP index was found to be 65.0% and 90.1% accurate, respectively, with a 95% CI of 0.804– 0.893. According to a stratified analysis, females, patients over the age of 56 and current nonsmokers were found to have a higher chance of developing NAFLD.
Conclusion: T2DM individuals with NAFLD were found to have a higher AIP index than those without NAFLD. The prevalence and progression of NAFLD in T2DM patients may be influenced by the AIP index.
Keywords: type 2 diabetes mellitus, nonalcoholic fatty liver disease, atherogenic index of plasma, diagnostic ability, cross-sectional study
Introduction
Fat accumulation in the liver is caused by complex interactions between hereditary factors and external factors, such as metabolic stress.1 A common chronic complication of diabetes mellitus is characterized as nonalcoholic fatty liver disease (NAFLD).2–5 NAFLD is a hepatic manifestation of metabolic syndrome that is typically linked to type 2 diabetes mellitus (T2DM), obesity, dyslipidemia, and other metabolic disorders.6–8 Increased obesity and diabetes incidence has led to a rise in the number of NAFLD problems in T2DM patients.9–11 According to the literature, the occurrence of NAFLD in diabetic patients is 40%-70%.6,12–15 The incidence of NAFLD was 27.85% in the T2DM group of a Chinese cohort study.16 Therefore, early prevention of the development of NAFLD in patients with T2DM is essential. The atherogenic index of plasma (AIP) is an efficient predictor of atherosclerosis and coronary heart disease risk.17,18 According to a number of studies, an elevated AIP index is linked to the development of diabetes, coronary heart disease, NAFLD, and hypertension, and it also has good predictive value.19–22 However, there is no research on the link between AIP and NAFLD in people with T2DM. Therefore, the aim of this study was to analyze the association between AIP and NAFLD in patients with T2DM and to investigate the potential of AIP as a potential risk factor for NAFLD.
Materials and Methods
Study Subjects
We screened 1074 patients from the Department of Endocrinology and Metabolism of the Affiliated Hospital of Southwest Medical University from March 2018 to May 2019. There were 542 males and 532 females aged 18 to 80 years, with an average age of 56.17 ± 11.54 years. Inclusion criteria: the American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” for diabetes mellitus, published in 2021, were completely met by all T2DM patients.23 Exclusion criteria: type 1 diabetes, specific types of diabetes due to other causes and gestational diabetes mellitus defined by the ADA “Standards of Medical Care in Diabetes” (2021);23 infectious diseases, severe liver insufficiency, severe cardiovascular diseases, psychiatric diseases, alcoholism and incomplete clinical data.
The study complied with the ethical standards of the Declaration of Helsinki (2013) and was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (file number: 2018017).24 The patients gave their informed consent.
Basic Information
Data regarding sex, age, duration of T2DM, and history of smoking (smoking defined as >1 cigarette/d, lasting >1 year) were collected. The physical examination indices were height, weight, waist circumference (WC), diastolic blood pressure (DBP), systolic blood pressure (SBP), heart rate, and body mass index (BMI). The laboratory test indices were fasting blood glucose (FBG), glycated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and γ-glutamyl transpeptidase (GGT) levels. After admission, all participants were given a light meal and fasted for at least eight hours at night. FBG, HbA1c, and lipid levels were determined by a fully automated biochemical analyzer using 5 ml of fasting venous blood obtained in the early morning of the next day.
AIP Assessment
AIP was determined as follows for each individual examined at the initial stage: log (TG (mmol/L)/HDL-c (mmol/L)).18 The AIP index was used to separate attendees into four groups: Q1 (<-0.1) (n=264), Q2 (0.1, 0.21) (n=273), Q3 (0.21, 0.43) (n=266), and Q4 (>0.43) (n=271).
Diagnosis of NAFLD
As a proxy of NAFLD, the fatty liver index (FLI) was utilized in this study. The NAFLD group was defined as having an FLI ≥ 60.25 The following formula was used to determine FLI: FLI=e(0.953 * ln (TG, mg/dL) + 0.139 * BMI (kg/cm2) + 0.718 * ln (GGT, mg/dL) +0.053 * WC -15.745)/(1 + e(0.953 * ln (TG, mg/dL) + 0.139 * BMI (kg/cm2) + 0.718 * ln (GGT, mg/dL) +0.053 * WC -15.745)) * 100.25
Statistical Analysis
The clinical features of the study subjects are reported as the means ± SDs. Categorical variables are numerically expressed (percent within group). To determine if the data followed a normal distribution, Q-Q plots were utilized. Independent samples t-test for normally distributed data, and nonnormally distributed data were tested by the Mann–Whitney U-test. Then, the χ2 test or Fisher’s exact test was used to evaluate group comparisons for categorical variables. We examined the anticipated accuracy of the AIP index using receiver operating characteristic (ROC) curves. SPSS.26.0 was utilized for every statistical analysis.
Results
Table 1 lists the characteristics of the 1074 patients in our study according to sex and NAFLD. The results showed that compared to non-NAFLD males, males with NAFLD were more likely to be middle-aged, to be current smokers, to use lipid-lowering drugs, to have higher values for BMI, waist circumference, diastolic blood pressure, heart rate, TC, TG, ALT, AST, GGT, FLI, AIP and to have lower values for HDL-c, LDL-c (all P<0.05). Similarly, females with NAFLD also had higher values for BMI, WC, DBP, heart rate, TC, TG, ALT, AST, GGT, FLI, and AIP and lower values for HDL-c and LDL-c (all P<0.05).
 |
Table 1 Baseline Clinical Data According to Gender and NAFLD Status |
Univariate regression analyses for all variables are presented in Table 2. According to the results, the prevalence of NAFLD was positively associated with sex, age, BMI, WC, DBP, heart rate, TC, TG, HDL, LDL, ALT, GGT, AIP, duration of diabetes, current smoking and use of lipid-lowering drugs (all P trend < 0.05). Next, variables that were not statistically significant in Table 2 were excluded, and those with multiple covariances (TG, HDL-c) were removed together to facilitate multiple regression analysis (Table 3). Model 1 was unadjusted for variables, and the odds ratio (OR) for each SD increase in AIP was 36.293. When adjusted for sex (Model 2), the OR for each SD increase in AIP was 32.284. After comprehensive adjustment for sex, age, BMI, WC, DBP, heart rate, TC, LDL, ALT, GGT, duration of diabetes, current smoking and lipid-lowering drugs (Model 3), the OR per SD increase in AIP was 2244.984. All of the models had an OR > 1, indicating that there was a strong positive association between AIP and NAFLD (all p<0.001). We transformed the AIP index to a categorical variable (AIP quartiles) to improve the study’s reliability, and the outcomes remained unchanged. Additionally, the higher the AIP index is, the greater the risk of NAFLD in the T2DM population.
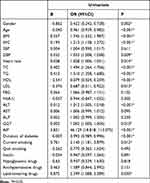 |
Table 2 Univariate Regression Analysis of Variables Contributing to NAFLD Prevalence |
 |
Table 3 Correlation Between NAFLD and AIP Quartiles |
The findings of the subgroup analysis are shown in Table 4. After excluding the factors causing multicollinearity, TG and HDL-c levels, the remaining effective variables were included in the binary regression analysis. There was a strong relationship between the performance of the AIP index and an elevated risk of NAFLD prevalence in females, patients ≥ 56 years of age, and current nonsmokers (all P for interaction < 0.05).
 |
Table 4 Association of AIP with NAFLD in Subgroup Analysis |
After that, we assessed the diagnostic value of the independent risk factors for NAFLD using an ROC curve (Figure 1). With an under the curve (AUC) of 0.891 (95% CI: 0.857, 0.925), WC was found to be the most accurate, and the second most accurate was the AIP index (AUC: 0.849, 95% CI: 0.804–0.893). By calculating the Jorden index, the optimum cutoff value of AIP was 0.56. The sensitivity of the AIP index was 65.0%, and the specificity was 90.1%.
 |
Figure 1 ROC curve of AIP index predicting NAFLD in patients with T2DM. |
Discussion
NAFLD is common in patients with type 2 diabetes, but there is still a lack of simple and easy predictors.3,13 This study proposes AIP as an assessment index to estimate the likelihood of developing NAFLD in T2DM patients and evaluated the diagnostic performance of the AIP index. After binary regression analysis, AIP was demonstrated to be a significant risk factor for concurrent NAFLD in patients with T2DM, and there was a trend for the prevalence of NAFLD to increase with increasing levels of AIP. Among T2DM individuals, females, patients >56 years of age, and current nonsmokers had a significantly increased risk of NAFLD. According to ROC curve analysis, the AIP index also had good diagnostic performance (AUC: 0.849, sensitivity: 65.0%, specificity: 90.1%).
Most studies have shown that the prevalence of NAFLD is higher in males than in females.26 This trend is reversed in postmenopausal females, possibly due to the loss of the protective effect of estrogen.27–29 The mean age of females with NAFLD in this study was 57 years old, which may explain the much higher prevalence of NAFLD in females with T2DM than in males in this study. A cross-sectional study from Peking Union Medical College Hospital showed that WC was the strongest predictor of NAFLD in postmenopausal females.30 This is consistent with our findings that WC performs best under the ROC curve. This adds strong scientific evidence to the increased prevalence of NAFLD in postmenopausal females and the migration of body fat to the abdomen.31,32 Insulin resistance was a common feature of all of the T2DM population included in this study, and insulin resistance indirectly contributes to the development of NAFLD.33
Smoking, a carcinogenic factor in liver cancer, also has a negative impact on the development of NAFLD; however, our findings show that current nonsmokers have a much higher risk than current smokers.34 We speculate that this result was found because these individuals were passive smokers or had quit smoking, and some studies show that quitting smoking may lead to NAFLD by increasing BMI.35 In addition, passive smoking caused by sidestream smoke is more harmful than active smoking.36,37
The development of T2DM can be predicted by the AIP index.38,39 Additionally, AIP was significantly correlated with the prevalence of NAFLD in both the obese and nonobese populations.40,41 On the basis of past research and the current data, it can be inferred that there is also a favorable correlation between AIP and NAFLD in patients with T2DM. The relationship between T2DM and NAFLD is characterized by a number of pathological alterations, including insulin resistance, an abnormal hepatic lipid profile that results in adiposity, and hyperinsulinemic dysfunction.42 This could be the reason why AIP is able to predict NAFLD in patients with T2DM.
The present cross-sectional study was derived from the community, ensuring the uniformity of personnel. And this is the first study to assess the relationship between AIP and NAFLD in T2DM patients. Some limitations exist for this study. Because our dataset lacked liver biopsies or imaging, we utilized the FLI index to characterize NAFLD, which is less accurate.43 In addition, this study was unable to obtain information on patient diets during their hospitalization to exclude the effect of diet on triglyceride production. Additionally, the study only sampled 1074 people in the Luzhou area, which is a small sample. A larger cross-sectional study or cohort study is needed to confirm the correlation.
Conclusion
In conclusion, a significantly increased AIP index was observed in patients with NAFLD and T2DM, and this association was stronger in women, those older than 56 years, and current nonsmokers. These findings suggest that the AIP index might have a substantial influence on the occurrence and development of NAFLD in T2DM patients. However, its mechanism of action remains unclear, and more studies are required to investigate the causal association.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University. Approval number: 2018017. Everyone involved in this study signed an informed consent form.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
A portion of this study was financed by the Chinese Ministry of Science and Technology grants 2016YFC0901200.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Michelotti A, de Scordilli M, Palmero L, et al. NAFLD-related hepatocarcinoma: the malignant side of metabolic syndrome. Cells. 2021;10(8):2034. doi:10.3390/cells10082034
2. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–2564. doi:10.1016/j.cell.2021.04.015
3. Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–544. doi:10.1016/j.jhep.2018.10.033
4. Stefan N, Häring H-U, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313–324. doi:10.1016/S2213-8587(18)30154-2
5. Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224. doi:10.1016/S0140-6736(20)32511-3
6. Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34(1):84–129. doi:10.1210/er.2012-1009
7. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi:10.1016/S2213-8587(14)70032-4
8. Ampuero J, Aller R, Gallego-Durán R, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48(11–12):1260–1270. doi:10.1111/apt.15015
9. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–430. doi:10.2337/dc16-1787
10. Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity. 2021;29(11):1950–1960. doi:10.1002/oby.23263
11. Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–296. doi:10.1016/S2213-8587(22)00003-1
12. Vieira Barbosa J, Lai M. Nonalcoholic fatty liver disease screening in type 2 diabetes mellitus patients in the primary care setting. Hepatol Commun. 2021;5(2):158–167. doi:10.1002/hep4.1618
13. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
14. Blachier M, Leleu H, Peck-Radosavljevic M, Valla D-C, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608. doi:10.1016/j.jhep.2012.12.005
15. Bril F, Cusi K. Nonalcoholic fatty liver disease: the new complication of type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2016;45(4):765–781. doi:10.1016/j.ecl.2016.06.005
16. Li Y, Wang J, Tang Y, et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS One. 2017;12(3):e0174291. doi:10.1371/journal.pone.0174291
17. Holmes DT, Frohlich J, Buhr KA. The concept of precision extended to the atherogenic index of plasma. Clin Biochem. 2008;41(7–8):631–635. doi:10.1016/j.clinbiochem.2008.01.023
18. Dobiásová M. AIP--aterogenni index plazmy jako vyznamny prediktor kardiovaskularniho rizika: od vyzkumu do praxe.. Vnitr Lek. 2006;52(1):64–71. Czech.
19. Won K-B, Heo R, Park H-B, et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis. 2021;324:46–51. doi:10.1016/j.atherosclerosis.2021.03.009
20. Hamzeh B, Pasdar Y, Mirzaei N, et al. Visceral adiposity index and atherogenic index of plasma as useful predictors of risk of cardiovascular diseases: evidence from a cohort study in Iran. Lipids Health Dis. 2021;20(1):82. doi:10.1186/s12944-021-01505-w
21. Fu L, Zhou Y, Sun J, et al. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):201. doi:10.1186/s12933-021-01393-5
22. Li Y-W, Kao T-W, Chang P-K, Chen W-L, Wu L-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11(1):9900. doi:10.1038/s41598-021-89307-z
23. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2021;44(Suppl1):S15–S33. doi:10.2337/dc21-S002
24. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
25. Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi:10.1186/1471-230X-6-33
26. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–1469. doi:10.1002/hep.30626
27. Wáng YXJ. Gender-specific liver aging and magnetic resonance imaging. Quant Imaging Med Surg. 2021;11(7):2893–2904. doi:10.21037/qims-21-227
28. DiStefano JK. NAFLD and NASH in postmenopausal women: implications for diagnosis and treatment. Endocrinology. 2020;161(10). doi:10.1210/endocr/bqaa134
29. Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(4):1406–1414. doi:10.1002/hep.26761
30. Liu PJ, Lou HP, Zhu YN. Identification of hepatic steatosis in premenopausal and postmenopausal women based on phenotypes combining triglyceride levels and anthropometric indices: a cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:1339–1347. doi:10.2147/DMSO.S302297
31. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32(6):949–958. doi:10.1038/ijo.2008.25
32. Park SH, Jeon WK, Kim SH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21(1 Pt 1):138–143. doi:10.1111/j.1440-1746.2005.04086.x
33. Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9(4):387. doi:10.3390/nu9040387
34. Lange NF, Radu P, Dufour J-F. Prevention of NAFLD-associated HCC: role of lifestyle and chemoprevention. J Hepatol. 2021;75(5):1217–1227. doi:10.1016/j.jhep.2021.07.025
35. Akhavan Rezayat A, Dadgar Moghadam M, Ghasemi Nour M, et al. Association between smoking and non-alcoholic fatty liver disease: a systematic review and meta-analysis. SAGE Open Med. 2018;6:2050312117745223. doi:10.1177/2050312117745223
36. Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005;14(6):396–404. doi:10.1136/tc.2005.011288
37. Liu Y, Dai M, Bi Y, et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. 2013;23(2):115–121. doi:10.2188/jea.JE20120067
38. Yi Q, Ren Z, Bai G, et al. The longitudinal effect of the atherogenic index of plasma on type 2 diabetes in middle-aged and older Chinese. Acta Diabetol. 2022;59(2):269–279. doi:10.1007/s00592-021-01801-y
39. Song P, Xu L, Xu J, et al. Atherogenic index of plasma is associated with body fat level in type 2 diabetes mellitus patients. Curr Vasc Pharmacol. 2018;16(6):589–595. doi:10.2174/1570161116666180103125456
40. Dong B-Y, Mao Y-Q, Li Z-Y, Yu F-J. The value of the atherogenic index of plasma in non-obese people with non-alcoholic fatty liver disease: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2020;19(1):148. doi:10.1186/s12944-020-01319-2
41. Xie F, Pei Y, Zhou Q, Cao D, Wang Y. Comparison of obesity-related indices for identifying nonalcoholic fatty liver disease: a population-based cross-sectional study in China. Lipids Health Dis. 2021;20(1):132. doi:10.1186/s12944-021-01560-3
42. Tanase DM, Gosav EM, Costea CF, et al. The intricate relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. doi:10.1155/2020/3920196
43. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209–1222. doi:10.1111/apt.12963
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
