Back to Journals » Clinical Ophthalmology » Volume 15
A Comparison of Refractive Accuracy Between Conventional and Femtosecond Laser Cataract Surgery Techniques Using Modern IOL Formulas
Authors Connell BJ, Kane JX, Vajpayee RB
Received 6 December 2020
Accepted for publication 15 February 2021
Published 2 March 2021 Volume 2021:15 Pages 899—907
DOI https://doi.org/10.2147/OPTH.S296032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Benjamin J Connell,1,2 Jack X Kane,2 Rasik B Vajpayee2– 4
1Eye Surgery Associates, Melbourne, Victoria, Australia; 2Corneal Unit, Royal Victorian Eye and Ear Hospital, Melbourne, Victoria, Australia; 3Centre for Eye Research Australia, University of Melbourne, Melbourne, Victoria, Australia; 4Vision Eye Institute, Melbourne, Victoria, Australia
Correspondence: Benjamin J Connell
Eye Surgery Associates, 2/232 Victoria Pde, East Melbourne, VIC, 3002, Australia
Tel +61 9416 0695
Fax +61 9416 1816
Email [email protected]
Purpose: To compare the refractive outcome prediction accuracy between conventional (CCS) and femtosecond laser assisted (FLACS) cataract surgery techniques using optimized lens constants for modern intraocular lens (IOL) formulas.
Patients and Methods: Our retrospective, comparative, interventional case series, compared data from 196 eyes undergoing CCS and 456 eyes undergoing FLACS with Acrysof IOL (Alcon laboratories, Inc) implantation. After optimizing IOL constants, the predicted refractive outcome was calculated for all formulas for each case. This was compared to the actual refractive outcome to provide the prediction error. The performance of CCS and FLACS was compared by the absolute prediction error and percentage of eyes within 0.25D, 0.5D and 1.0D of anticipated refractive outcome.
Results: There was no statistically significant difference in median absolute error between the CCS and LACS groups for the Kane (0.256, 0.236; p=0.389), SRK T (0.298, 0.302, p=0.910), Holladay (0.312, 0.275; p=0.090), Hoffer Q (0.314, 0.289; p=0.330), Haigis (0.309, 0.258; p=0.177), Barrett Universal 2(0.250, 0.250; p=0.866), Holladay 2 (0.250, 0.258; p=0.860) and Olsen (0.260, 0.255; p=0.570) formulas. Similarly, there was no consistent difference between the two techniques for percentage of patients within 0.25, 0.50 and 1.0D of predicted refractive outcome for each formula.
Conclusion: There was no difference in refractive outcome prediction accuracy between the CCS and FLACS techniques.
Keywords: femtosecond laser-assisted cataract surgery, refractive predictability, IOL formulas
Introduction
Recent generation of IOL formulas have significantly improved the refractive outcome prediction accuracy of modern cataract surgery. The ability of modern IOL formulas to achieve superior refractive outcomes over earlier third generation formulas1–3 has been possible due to their accuracy at predicting the effective lens position. Recent large studies4–7 comparing CCS and FLACS have demonstrated no clear differences in prediction of refractive accuracy using earlier IOL formulas. However, none of these studies undertook the recommended8 IOL constant optimization to eliminate the source of bias inherent to IOL constants recommended by manufacturers.
We postulated that the improved precision of modern formulas, together with IOL constant optimization, may demonstrate that the superior capsulotomy geometry and IOL position9,10 advantages with FLACS translates into improved refractive outcomes.
In the present study, we investigated whether refractive outcome predictions were more accurate in FLACS when compared with CCS using optimized IOL constants for modern IOL formulas.
Patients and Methods
This retrospective, comparative, interventional comparative case series included all patients that had undergone CCS or FLACS surgery performed by a single surgeon (BC) between July 2015 and July 2019. Patients who had co- morbidities such as corneal scarring or previous ocular surgery were excluded. Other exclusion criteria were occurrence of intraoperative or postoperative complications or patients who had post-operative vision less than 6/12 (20/40) equivalent.
If both eyes from a single patient met the inclusion criteria, one eye was randomly chosen for inclusion in the analysis. The study was approved by the Royal Victorian Eye and Ear Hospital Human Research Ethics Committee. Individual patient consent was not required as no patient identifying data was stored and retrospective study design, in compliance with the local data privacy laws. The study was conducted in compliance with the Declaration of Helsinki.
Preoperative and Postoperative Examinations
Patients underwent a preoperative full visual acuity assessment, slit lamp anterior and posterior segment examination. Preoperative biometry was performed using the IOLMaster model 700 (Carl Zeiss Meditec AG).
Group Allocation
The population typically held private health insurance and each patient self-selected either technique based on their own preference, considering the increased out of pocket expense for the laser assisted technique.
Surgical Techniques
The same surgeon (BC) performed all surgeries in a private operating facility under topical anesthesia. Capsulotomies were centered on the pupil. After removal of the cataract, an Alcon SN60WF or Alcon T6 series (Alcon Laboratories, Fort Worth Texas) IOL was injected. The wound was enlarged only for higher powered IOLs, as per the manufacturer’s recommendation. Postoperative management was identical for the two groups. Prednisolone acetate 1% (Prednefrin Forte, Allergan) and Chloramphenicol 0.5% (Chlorsig, Sigma Pharmaceuticals, Australia) were used four times per day for 4 weeks following surgery.
Conventional Technique
In this technique, clear corneal temporal 2.4mm wounds were used and the capsulotomy performed using forceps, followed by traditional phacoemulsification.
Femtosecond Laser Technique
The laser (LenSx platform, Alcon Surgical Inc) was used to perform the capsulotomy, lens fragmentation and wound construction. The pupil centered capsulotomy was used with the following parameters: 4.9mm diameter, with delta up 270 µm, delta down 330 µm, spot energy 6.50 µJ, spot separation 4µm and layer separation 4µm. Traditional phacoemulsification was then used to remove the nucleus.
Formulas to Predict Post-Operative Spherical Equivalent Outcome
Haigis,11 Hoffer Q,12,13 Holladay14 and SRK/T15 formulas were programmed into a previously validated16 Excel spreadsheet (Microsoft, Redmond, Washington, USA) using the original publications and errata.
Data was entered into the respective third-party calculators for the other formulas: PhacoOptics program for the Olsen formula,17 IOL Consultant software for the Holladay 2 formula,18 and online calculators for the Barrett Universal 2.19 The Kane formula was calculated by one of the authors (JK).
The constant for each formula was optimized to produce a mean prediction error of zero (or as close as possible) by performing multiple iterations of the data using varying constants. For the Haigis formula, results were included for single (a0) and triple constant optimization.
For some formulas, a mean prediction error of zero could not be obtained due to limitations in how many decimal places could be entered for the constant into the calculator. In these cases the small residual mean error was removed by adjusting the refractive prediction error for each eye by an amount equal to the mean prediction error in that group as described in the JCRS editorial by Wang et al.20
Statistical Analysis
All statistical analysis was performed using Stata IC version 14 (College Station, TX, USA). Categorical variables were compared between surgical technique groups with Fisher’s exact test. The distribution of continuous variables was assessed with the Shapiro–Wilk normality test and then the CCS and FLACS groups were compared using the two-sample t-test for normally-distributed variables and the Wilcoxon rank-sum test if the variable was not normally distributed.
Results
During the study period 352 CCS and 826 FLACS procedures were performed. After exclusions, 196 eyes (from 196 patients) were included in the CCS group and 456 eyes (from 456 patients) in the FLACS group. Details of the exclusions are show in Table 1.
 |
Table 1 Indications for Subject Exclusion from the Analysis |
Small, statistically significant differences were noted in the median for baseline demographics between the two groups (Table 2). The CCS group was older (75 v 73 years; p=0.008), had shorter axial length (23.33 v 23.66mm; p=0.028), anterior chamber depth (3.03 v 3.14mm; p=0.002), thicker lens (4.71 v 4.59mm; p=<0.001) and inferior post-operative corrected distance visual acuity (0.00 v 0.00; p = 0.005).
 |
Table 2 Baseline Demographic and Clinical Characteristics |
There was no statistically significant difference in gender proportions (37/40% Male) or toric IOL use (71% for both) between the CCS/FLACS groups. The groups displayed similar distributions of certain comorbidities (Table 3). Optimized constants are shown in Table 4.
 |
Table 3 Comorbidity Counts |
 |
Table 4 Constants Used for the Different Formulas |
There was no statistically significant difference in the median absolute error between the CCS and FLACS groups for any of the formulas (Table 5). There was also no difference in percentage of patients within 0.25, 0.50 and 1.00 D of predicted refraction for the CCS compared to the FLACS group (Table 6) for any of the formulas with the only exception the single constant optimized Haigis formula, where the percentage achieving within 1.0D of predicted was statistically higher in the FLACS group (98.5% v 95.9%; p=0.047).
 |
Table 5 Prediction and Absolute Errors for Each Formula |
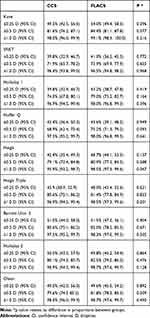 |
Table 6 % of Eyes Within 0.25, 0.50 and 1.0D of Absolute Error |
Discussion
A femtosecond capsulotomy used in FLACS is considered geometrically superior to the manual capsulotomy in CCS. Studies have demonstrated it to have a more predictable diameter,9,21–24 more circular,9,21–25 less eccentric,9,23,24 and less shrinkage post-operatively.21,26 Consequently, the IOL position in FLACS demonstrates less tilt,10 decentration,10 greater overlap of the optic9 and the post-operative IOL anterior-posterior position deviates less from predicted.21
Effective lens position has been shown to be the most important factor in refractive prediction accuracy.27 We hypothesized, that the superior FLACS capsulotomy geometry when compared with CCS, might translate improved in refractive accuracy by improving the predictability of the effective lens position.
Clinical studies to date have typically used 3rd generation formulas and not consistently demonstrated a refractive benefit for FLACS. The FEMCAT,4 FACT5 and Roberts28 randomized control trials, a prospective intraindividual trial,29 the EUREQUO registry30 and retrospective studies by Berk6 and Chee,7 have all used 3rd generation formulas and relatively large numbers have not demonstrated any refractive advantage. Ewe and colleagues,31 in a prospective, non-randomized comparative study demonstrated an advantage for CCS over FLACS.
Only a few smaller studies, also using 3rd generation formulas, have identified a refractive advantage for FLACS. A prospective study of 132 eyes32 published in 2012 reported the mean absolute error was less with FLACS (0.5/0.38D, p=0.04). A prospective intraindividual study by Conrad-Hengerer33 with one eye of 100 patients randomized to each technique published in 2015 demonstrated 71/92% (p<0.05) of eyes within 0.5 D of the intended outcome for CCS/FLACS. An accuracy of 92% of eyes achieving within 0.5D for FLACS demonstrated in the Conrad-Hengerer study is relatively high and has not been replicated in other studies. For example, our study with modern biometry, optimized constants and strict case exclusion criteria demonstrated only 72/74% (SRK/T) and 79/81% (Haigis) from the CCS/FLACS groups were within 0.5D of the intended outcome. Other large studies5,6,28,31,34 have typically reported 75% or less and no more than 83%, in either group, achieving within 0.5D of the intended outcome.
More recent relatively small studies, also using older 3rd formulas, have only demonstrated a benefit for FLACS on some outcome measures. A recent retrospective study with 50 cases in each group, reported a significantly greater percentage of eyes within 0.5D of the intended outcome (48/76%, p = 0.01) but no difference between groups for the mean absolute error. In this study, there was a mean prediction error difference between the groups (−0.42/-0.11) because they did not optimize their lens constants. This difference in mean prediction error likely explains the difference in mean absolute errors reported. A large retrospective comparative case series with 3144 eyes34 demonstrated a statistically significant lower mean absolute error for FLACS compared with CCS (0.60 v 0.54D) however there was no difference in percentage of patients within 0.5D of intended.
None of these studies had used modern IOL formulas such as the Barrett Universal 2, Olsen or Kane formulas which have been reported to be more accurate than third generation formulas1–3 to predict refractive accuracy. To the best of our knowledge, the only study using a later generation formula, was an intraindividual RCT of 110 paired eyes by Dzhaber35 which used the Holladay 2 formula and did not demonstrate any difference. With this formula they reported similar values to other studies with 81/84% (p=0.17) of cases within 0.5D of the anticipated outcome. We hypothesized that when these modern formulas are used, there may be a refractive benefit for FLACS not evident with third generation formulas.
Ours is the first study that compared refractive prediction outcomes of FLACS and CCS using IOL constant optimization and included only one eye per patient as per the published recommendation.8 The recommendation to perform IOL constant optimization allows a more reliable comparison between formulas, with any difference in absolute error likely to reflect a true difference in formula accuracy. However, despite optimizing IOL constants in our study, the refractive accuracy was very similar to large prospective FEMCAT4 and FACT5 trials which did not perform optimization. Results of our study reconfirms that although the capsulotomy performed by FLACS may appear much more central and circular as compared to CCS, it does not translate in to better ELP and achieving more accurate refractive outcomes.
FLACS cases have also been demonstrated to show less capsular bag26 and capsulotomy shrinkage21 at 1–3 months. It is also possible that these longer-term shrinkage forces are distributed relatively less symmetrically to the more irregular, less centered CCS capsulotomy and therefore more likely to induce long term IOL tilt, decentration and refractive change. This may be beneficial in the longer term for patients with multifocal and extended depth of focus lenses where lens tilt and decentration would degrade the visual outcome. A study using these lenses with longer term follow up would help address this question. A recent meta-analysis and commentary36,37 reported a clinically but not statistically significant lower rate of posterior capsule rupture with LACS, which would also benefit refractive outcome since IOL position is less predictable in these cases.
Overall, our study did not find any refractive advantage for FLACS over CCS when using modern IOL formulas and optimized IOL constants during a short-term follow-up. This confirms that a refractive advantage should not be used in guiding a patient’s decision to proceed with either technique.
A disadvantage of our study is the potential for bias associated with patient self-selection for the either procedure. FLACS incurs an increased patient out of pocket expense in Australia of $AUD850 (equivalent to $US550 or 500euros).
The strengths of this study include that the surgeries were performed by a single surgeon, consistent staff performed the follow up assessments and modern biometry (IOLMaster model 700) was used. In addition, few patients were lost to follow up, the series was relatively large, and a systematic approach taken to case exclusion.
Conclusion
Our study found no difference in refractive outcome prediction accuracy between the CCS and FLACS techniques using modern IOL formulas and optimized constants.
Acknowledgments
Sophie Rogers provided statistical support.
Disclosure
Jack X. Kane is the owner of the Kane formula. The authors report no other conflicts of interest in this work.
References
1. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125:169–178. doi:10.1016/j.ophtha.2017.08.027
2. Melles RB, Kane JX, Olsen T, Chang WJ. Update on intraocular lens calculation formulas. Ophthalmology. 2019;126:1334–1335. doi:10.1016/j.ophtha.2019.04.011
3. Darcy K, Gunn D, Tavassoli S, Sparrow J, Kane JX. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10 930 eyes from the UK National Health Service. J Cataract Refract Surg. 2020;46:2–7. doi:10.1016/j.jcrs.2019.08.014
4. Schweitzer C, Brezin A, Cochener B, et al. Femtosecond laser-assisted versus phacoemulsification cataract surgery (FEMCAT): a multicentre participant-masked randomised superiority and cost-effectiveness trial. Lancet. 2020;395:212–224. doi:10.1016/S0140-6736(19)32481-X
5. Day AC, Burr JM, Bennett K, et al. Femtosecond laser-assisted cataract surgery versus phacoemulsification cataract surgery (FACT): a randomized noninferiority trial. Ophthalmology. 2020;127:1012–1019. doi:10.1016/j.ophtha.2020.02.028
6. Berk TA, Schlenker MB, Campos-Moller X, Pereira AM, Ahmed IIK. Visual and refractive outcomes in manual versus femtosecond laser-assisted cataract surgery: a single-center retrospective cohort analysis of 1838 eyes. Ophthalmology. 2018;125:1172–1180. doi:10.1016/j.ophtha.2018.01.028
7. Chee SP, Yang Y, Ti SE. Clinical outcomes in the first two years of femtosecond laser-assisted cataract surgery. Am J Ophthalmol. 2015;159:714–719. doi:10.1016/j.ajo.2015.01.016
8. Hoffer KJ, Aramberri J, Haigis W, et al. Protocols for studies of intraocular lens formula accuracy. Am J Ophthalmol. 2015;160:403–405 e1. doi:10.1016/j.ajo.2015.05.029
9. Nagy ZZ, Kranitz K, Takacs AI, Mihaltz K, Kovacs I, Knorz MC. Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J Refract Surg. 2011;27:564–569. doi:10.3928/1081597X-20110607-01
10. Kranitz K, Mihaltz K, Sandor GL, Takacs A, Knorz MC, Nagy ZZ. Intraocular lens tilt and decentration measured by Scheimpflug camera following manual or femtosecond laser-created continuous circular capsulotomy. J Refract Surg. 2012;28:259–263. doi:10.3928/1081597X-20120309-01
11. Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–773. doi:10.1007/s004170000188
12. Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19:700–712. doi:10.1016/S0886-3350(13)80338-0
13. Zuberbuhler B, Morrell AJ. Errata in printed Hoffer Q formula. J Cataract Refract Surg. 2007;33:
14. Holladay JT, Prager TC, Chandler TY, Musgrove KH, Lewis JW, Ruiz RS. A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg. 1988;14:17–24. doi:10.1016/S0886-3350(88)80059-2
15. Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16:333–340. doi:10.1016/S0886-3350(13)80705-5
16. Kane JX, Van Heerden A, Atik A, Petsoglou C. Intraocular lens power formula accuracy: comparison of 7 formulas. J Cataract Refract Surg. 2016;42:1490–1500. doi:10.1016/j.jcrs.2016.07.021
17. Olsen T. PhacoOptics. Available from: https://www.phacooptics.net/. Accesed October 1, 2019.
18. Holladay JT. Holladay IOL Consultant Software & Surgical Outcomes Assessment. Bellaire, TX: Holladay Consulting; 2015.
19. Barrett GD. Barrett Universal II Formula. Singapore: Asia-Pacific Association of Cataract and Refractive Surgeons; 2018.
20. Wang L, Koch DD, Hill W, Abulafia A. Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J Cataract Refract Surg. 2017;43:999–1002. doi:10.1016/j.jcrs.2017.08.003
21. Panthier C, Costantini F, Rigal-Sastourne JC, et al. Change of capsulotomy over 1 year in femtosecond laser-assisted cataract surgery and its impact on visual quality. J Refract Surg. 2017;33:44–49. doi:10.3928/1081597X-20161028-01
22. Friedman NJ, Palanker DV, Schuele G, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37:1189–1198. doi:10.1016/j.jcrs.2011.04.022
23. Mastropasqua L, Toto L, Mattei PA, et al. Optical coherence tomography and 3-dimensional confocal structured imaging system-guided femtosecond laser capsulotomy versus manual continuous curvilinear capsulorhexis. J Cataract Refract Surg. 2014;40:2035–2043. doi:10.1016/j.jcrs.2014.05.032
24. Kranitz K, Takacs A, Mihaltz K, Kovacs I, Knorz MC, Nagy ZZ. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27:558–563. doi:10.3928/1081597X-20110623-03
25. Ostovic M, Klaproth OK, Hengerer FH, Mayer WJ, Kohnen T. Light microscopy and scanning electron microscopy analysis of rigid curved interface femtosecond laser-assisted and manual anterior capsulotomy. J Cataract Refract Surg. 2013;39:1587–1592. doi:10.1016/j.jcrs.2013.07.024
26. Dick HB, Conrad-Hengerer I, Schultz T. Intraindividual capsular bag shrinkage comparing standard and laser-assisted cataract surgery. J Refract Surg. 2014;30:228–233. doi:10.3928/1081597X-20140320-01
27. Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34:368–376. doi:10.1016/j.jcrs.2007.10.031
28. Roberts HW, Wagh VK, Sullivan DL, et al. A randomized controlled trial comparing femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery. J Cataract Refract Surg. 2019;45:11–20. doi:10.1016/j.jcrs.2018.08.033
29. Krarup T, Ejstrup R, Mortensen A, la Cour M, Holm LM. Comparison of refractive predictability and endothelial cell loss in femtosecond laser-assisted cataract surgery and conventional phaco surgery: prospective randomised trial with 6 months of follow-up. BMJ Open Ophthalmol. 2019;4:e000233. doi:10.1136/bmjophth-2018-000233
30. Manning S, Barry P, Henry Y, et al. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 2016;42:1779–1790. doi:10.1016/j.jcrs.2016.10.013
31. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123:178–182. doi:10.1016/j.ophtha.2015.09.026
32. Filkorn T, Kovacs I, Takacs A, Horvath E, Knorz MC, Nagy ZZ. Comparison of IOL power calculation and refractive outcome after laser refractive cataract surgery with a femtosecond laser versus conventional phacoemulsification. J Refract Surg. 2012;28:540–544. doi:10.3928/1081597X-20120703-04
33. Conrad-Hengerer I, Al Sheikh M, Hengerer FH, Schultz T, Dick HB. Comparison of visual recovery and refractive stability between femtosecond laser-assisted cataract surgery and standard phacoemulsification: six-month follow-up. J Cataract Refract Surg. 2015;41:1356–1364. doi:10.1016/j.jcrs.2014.10.044
34. Nithianandan H, Jegatheeswaran V, Dalal V, et al. Refractive laser-assisted cataract surgery vs. conventional manual surgery: comparing efficacy and safety in 3144 eyes. Am J Ophthalmol. 2019.
35. Dzhaber D, Mustafa OM, Alsaleh F, Daoud YJ, Daoud YJ. Visual and refractive outcomes and complications in femtosecond laser-assisted versus conventional phacoemulsification cataract surgery: findings from a randomised, controlled clinical trial. Br J Ophthalmol. 2020;104:225–229. doi:10.1136/bjophthalmol-2018-313723
36. Kolb CM, Shajari M, Mathys L, et al. Comparison of femtosecond laser-assisted cataract surgery and conventional cataract surgery: a meta-analysis and systematic review. J Cataract Refract Surg. 2020;46:1075–1085. doi:10.1097/j.jcrs.0000000000000228
37. Levitz LM, Wendell JS, Lawless M, Dick HB, Nagy Z. Comment on: comparison of femtosecond laser-assisted cataract surgery and conventional cataract surgery and conventional cataract surgery: a meta-analysis and systematic review. J Cataract Refract Surg. 2021;47:278. doi:10.1097/j.jcrs.0000000000000534
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
