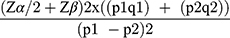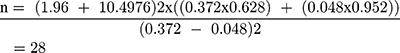Back to Journals » Open Access Surgery » Volume 14
A Comparison of Awake Versus Deep Removal of Laryngeal Mask Airway in Children Aged 2 to 8 Years Who Underwent Ophthalmic Procedures at Menilik II Hospital: A Prospective Observational Cohort Study
Authors Hika A , Ayele W , Aberra B , Aregawi A , Bantie AT, Mulugeta S , Chemeda D, Seifu A
Received 19 October 2020
Accepted for publication 10 February 2021
Published 10 March 2021 Volume 2021:14 Pages 9—15
DOI https://doi.org/10.2147/OAS.S287507
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Luigi Bonavina
Assefa Hika,1 Wubishet Ayele,2 Bacha Aberra,2 Adugna Aregawi,3 Abere Tilahun Bantie,4 Sintayehu Mulugeta,5 Dinkisisa Chemeda,6 Ashenafi Seifu3
1Department of Anesthesiology, College of Health Science, Aksum University, Aksum, Ethiopia; 2Department of Anesthesiology, Harar Health Science College, Harar, Ethiopia; 3Addis Ababa University, School of Medicine, Department of Anesthesia, Addis Ababa, Ethiopia; 4Department of Anesthesiology, College of Medicine and Health Science, Addigrat University, Addigrat, Ethiopia; 5Department of Anesthesiology, College of Health Science, Mekelle University, Mekelle, Ethiopia; 6Department of Anesthesiology, College of Health Science, Arsi University, Oromiya, Ethiopia
Correspondence: Abere Tilahun Bantie
Department of Anesthesiology, College of Medicine and Health Science, Addigrat University, PO Box: 50, Addigrat, Ethiopia
Tel +251(0)920442222
Email [email protected]
Background: Laryngeal mask airway (LMA) is a useful airway device which provides an alternative to ventilation through a face mask or endotracheal tube during ophthalmic surgery. It can be removed either when a child awakens or deeply anesthetized. But there is little evidence for best practice on the timing of their removal after ophthalmic surgery in the pediatric population. It has been studied by various investigators but with conflicting results and conclusions.
Objective: The aim of this study was to evaluate the effect of depth of anesthesia (awake or deep anesthesia) on the incidence of airway associated complications during LMA removal.
Methodology: A prospective observational cohort study was conducted from January to April 2018. Sixty-two American Society of Anesthesiologists physical status I and II pediatric (aged 2– 8 years) patients who underwent ophthalmic procedures under general anesthesia with LMA were recruited. Grouping (awake group or deep group) was done based on independent decision of on duty anesthetist and halothane 1– 1.5% was used as maintenance anesthesia. The incidence of airway-related adverse events like coughing, upper airway obstruction (Laryngospasm), breath holding, desaturation, excessive salivation, biting, vomiting, and retching with LMA removal were evaluated. Collected data were entered into Epi Info™ version 7.0 and transported to the SPSS version 22 for analysis. Fisher’s exact test and Chi-square test were used to analyze dependent variables and P-values less than 0.05 were considered statistical significance.
Results: There were no significant differences in airway-related adverse events. The incidence of coughing (12.9%, 6.5%), upper airway obstruction (41.9%, 35.5%), breath holding (9.7%, 3.2%), desaturation (16.1%, 22.6%), excessive salivation (19.4%, 12.9%), and biting (6.5%, 0%) between awake and deep groups respectively with (p > 0.05). Laryngospasm, vomiting, and retching did not occur in either group.
Conclusion: There was no significant difference in the incidence of airway-related adverse events whether the LMA was removed in a deep or awake condition.
Keywords: airway-related adverse events, laryngeal mask airway removal, LMA, awake/deep, ophthalmic procedures, pediatric patients, Ethiopia
Introduction
Establishing, securing, and maintaining a patent airway is a must during surgical procedure under general anesthesia.1 Mask ventilation and endotracheal intubation has been the foundation of airway management before laryngeal mask airway (LMA) emerged as one of the most important developments in airway devices.1
LMA is a pivotal component of the American Society of Anesthesiologist (ASA) Difficult Airway Algorithm.2,3 It provides a safe and effective form of airway management in children.4 Aspiration remains a serious and even fatal consequence of LMA use4 It is considered as more stimulating than afacemask but certainly less than the ETT.5
Induction and emergence from anesthesia are the most critical periods. However, some authors have observed that emergence from anesthesia tends to become the most critical period, possibly in relation to changes in practice including the use of LMA and/or of propofol and newer inhalational agents.6
Removal of airway devices from patient’s airway is associated with higher incidence of respiratory complications compared to the incidence of adverse events (coughing, straining, bronchospasm, laryngospasm, vomiting) occur during the time of insertion.7,8
Use of a potentially irritant volatile anesthetic such as desflurane or isoflurane, the presence of secretions or blood in the airway, and multiple instrumentation of the airway at light planes of anesthesia are potential anesthetic factors that increases risk of laryngospasm in combination with patient or surgical related causes.9
The use of the LMA and the inexperience of the anesthetist, especially when dealing with children, have been associated with a greater incidence of laryngospasm. On the other hand, use of intravenous anesthetic agents has been associated with a lower incidence of laryngospasm.9
Children are more prone to laryngospasm than adults, with laryngospasm being reported more commonly in children (17.4/1000) than in the general population (8.7/1000).2,5–7 In fact, the incidence of laryngospasm has been found to range from 1/1000 up to 20/100 in high-risk surgery.10
Laryngospasm can result in life-threatening complications including severe hypoxia, bradycardia, negative pressure pulmonary edema, and cardiac arrest. It remains the leading cause of perioperative cardiac arrest of respiratory origin in children.5
The optimal time for removing LMA is not known exactly.8,11,12 Manufacturers designer of the LMA suggest that LMA should be removed in awaked state.13–15 Some studies recommend that removal of LMA in the deep patients was associated with less adverse respiratory effects16 while another scholars also conclude that there was no difference in the incidence of airway complications whether the LMA was removed in the anesthetized or the awake patients.14 Because of these contradicting results anesthetists choose either one based on their experience. Thus, the aim of this study was to evaluate the effect of depth of anesthesia on respiratory adverse effects after LMA removal.
Methods
Study Setting
The study was conducted at Minilik II Hospital. The hospital is one of largest hospital in Addis Ababa. It provides comprehensive health services to patients who come from all parts of the country. The hospital is well known by ophthalmic surgery. There are six operation tables in the ophthalmic operation department from which one is always reserved for pediatric procedure. This manuscript is a thesis work for partial fulfillment of MSC degree in anesthesia.17
Study Design and Period
A prospective observational cohort study was conducted from January 15 to April 05/2018.
Source Population
All pediatric patients who underwent ophthalmic surgery under LMA at Minilik II Hospital.
Study Population
All pediatric patients scheduled for ophthalmic surgery from January 15 to April 05/2018.
Study Variables
Dependent variables were airway-related adverse events while independent variables include age, sex, weight, anesthesia condition (awake/deep) for removal of LMA.
Inclusion Criteria
Inclusion criteria includes all pediatric patients, American Society of Anesthesiologists physical status (ASA I and II), aged between 2 and 8 years, ophthalmic surgery under general anesthesia with LMA and use halothane as maintenance anesthesia.
Exclusion Criteria
Pediatric patients with active upper and lower respiratory tract infection within 2–4 weeks, airway disease (Asthma, COPD), more than three attempts of LMA insertion, an anticipated difficult airway, duration of surgery above 60 minutes, and administration of any drug that affects the airway during anesthesia were considered as exclusion criteria.
Sample Size Determination
The sample size for the study was calculated using double population proportion formula for comparison of two proportions based on the following assumptions: significance level 5% (α= 0.05), power of study (1 – β) of 90%. According to a study published in 2012 the incidence of airway-related adverse events in awake and deep removal were 37.2% and 4.8%, respectively.6,18 Taking 37.2% as P1 and 4.8% as P2, the calculation of sample size became:
Adding 10% for accidental withdrawal from the study, n became 31.
So, total sample size become 31 × 2 = 62.
Sampling Technique
After approval of ethical clearance by the ethical review committee, Addis Ababa University and receiving permission letter from Menelik-II hospital 62 patients were enrolled in this study.
Patients were categorized as awake removal group and deep removal group based on independent decision of responsible anesthetist. Participants were consecutively selected for each group till the required sample sizes were achieved.
Anesthesia Management Standard Protocols
Anesthetic management was standardized according to the hospital protocol. The study protocol was approved by ethical review committee. Then written and informed consent was obtained from parents or guardians. The study was conducted in accordance with the Declaration of Helsinki.19
Preanesthetic evaluation was done the day before surgical procedure was undertaken. All patients were nothing per mouth (NPO) since mid-night for 8 hours as per NPO Guidelines20 and did not get any sedative or effective drug on the airway preoperatively.
Preinduction basic intraoperative monitoring consisting of electrocardiogram (ECG), noninvasive blood pressure (NIBP) cuff, pulse oximeter and capnograph were used in all patients. Two minutes before induction of anesthesia, 1.5 μg/kg of fentanyl, 1 μg/kg of dexmedetomidine and1.5 mg/kg of lidocaine were administered intravenously. After preoxygenation, induction of anesthesia was performed with propofol 3mg/kg. 1.5% MAC of halothane was administered until adequate jaw relaxation was attained, and then appropriate LMA size was inserted by MSc anesthetists based on insertion method described in the manufacturer’s instruction manual. Anesthesia was maintained by 1.5% MAC of halothane in both groups. Opioid analgesics were not administered during surgery.
At the end of surgery, patients were randomly assigned to either the awake group (Group “A”) or the deep anesthesia group (Group “B”) by responsible anesthetist. LMA removal was done according to group-specific guidelines.21
In Group“A” (awake), halothane was stopped and 6 L/min of 100% oxygen was given to wash out the effect halothane. LMA was removed when patients meet the recovery criteria including facial grimace, spontaneous eye opening, and purposeful arm movement and responding to verbal commands in older patients.
In Group “B”, the LMA was removed while anesthesia was maintained with 1% of halothane and adequate spontaneous ventilation (symmetric chest expansion, adequate volume of gas in the reservoir bag volume, SpO2 > 95% and end-tidal CO2, within 35–45mmHg) was confirmed. Immediately after LMA removal halothane was stopped.
After removal of the LMA, all participants were transferred to post anesthesia care unit with 6 L/min of 100% O2 through tightly fitted facemask. Pain was controlled with 125 to 250 mg paracetamol per rectum according to weight of patient.
An awake state was defined as return of airway reflexes, purposeful movement and eye opening. A deeply anaesthetized state was defined as recovery of spontaneous ventilation but depressed airway reflexes with age-appropriate minimum alveolar concentration of halothane.
Data Collection Procedure
Each participant and grouping was coded to avoid bias. Then on duty anesthetists were given the data collection format to collect data blindly. The first data recorded preoperative information like patient’s age, sex, ASA status, BMI, LMA size were recorded from patent chart and anesthesia record.
The second data collector recorded frequency of airway-related complications including coughing, breath holding, laryngospasm, desaturation (SpO2< 95%), excessive secretions, LMA biting and vomiting, were recorded during emergence and 10 minutes after LMA removal. The frequency of upper airway obstruction requiring the use of airway adjuncts or airway support for both chin lift and jaw thrust also recorded.
Data Quality Assurance
To assure the quality of data, a pretest was done on 5% of the sample size. Data collectors were trained on inclusion and exclusion criteria, how to approach the study participants and how to use data collection tools.
In addition, regular checkup for completeness and consistency of the data was made on daily basis by the investigator.
Data Processing and Analysis
The collected data was entered into Epi Info™ version 7.0, and exported to Statistical Package for the Social Sciences (SPSS) version 20 computer program for analysis. Descriptive statistics were summarized in texts, tables.
Patient characteristics were analyzed using independent t-test and Chi-square test, The airway-related adverse events such as breath holding, coughing, and LMA biting were analyzed using Fisher’s exact test while upper airway obstruction, excessive salivation, and desaturation were analyzed using Chi-square test. P value less than 0.05 (p < 0.05) was considered as statistically significant.
Anesthesia Management Standard Protocols
Anaesthetic management was standardized according to the following protocol. Monitoring was applied preinduction and included an ECG, pulse oximeter, capnograph, and NIBP monitor.
Results
Socio-Demographic and Operative Characteristics
A total of 62 children who underwent ophthalmic surgery with LMA were involved in the study. Majority of the participants were male (41). Patient Characteristics were comparable in both groups with p> 0.05.
The mean age of respondents for awake LMA removal group was 4.9 ± 1.7 and the mean age of respondents for deep LMA removal group was 4.7 ± 1.8.
It was also found that the mean weight of patients was 20.5 ± 5.8 vs. 18.8 ± 5.9 in awake and deep LMA removal groups respectively (Table 1). Among ophthalmic surgical procedures small incision cataract surgery and corneoscleral repair were done for children 12 (19.35%), 11 (17.75%) respectively (Table 2).
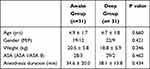 |
Table 1 Patient Characteristics |
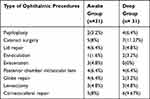 |
Table 2 Common Ophthalmology Surgical Procedures from January 15 to April 05/2018 |
Airway-Related Adverse Events
LMA removal associated adverse effects were noted in 37 patients. Of these 22 patients manifested adverse events in awake state, while 15 patients encountered adverse events in deep condition. Upper airway obstruction was seen in 24 patients, 13 from the awake group and 11 from the deep group. But all of these cases relieved by jaw thrust or chin lift maneuver. Twelve patients developed desaturation (SpO2 less than 90%), five in the awake group and seven in the deep group. However, there was no significant difference in the incidence of airway complications between the groups (p >0.05). Laryngospasm, vomiting, and retching did not occur in either group (Table 3).
 |
Table 3 Number of Patients with Airway-Related Adverse Effects from January 15 to April 05/2018 |
Percent Incidence of Airway-Related Adverse Events
Upper airway obstruction, coughing, breathing hold, excessive salivation and LMA biting were observed more frequently in awake group while desaturation was common in deep LMA removal group (Table 3)
Discussion
Pediatric ophthalmic surgery usually requires general anesthesia and tracheal intubation that may have deleterious effects on cardiovascular function.22,23 LMA has been found to be superior to tracheal intubation in terms of maintaining stable vital signs8 but positive pressure ventilation could become a challenge in certain cases. LMA offers the advantage of providing a better seal in the oropharynx to allow ventilation at much higher pressure and to protect the stomach from gastric insufflations.14
Inhalation induction remains a widely used technique in pediatrics and small children who are difficult to cannulation. Particularly halothane and sevoflurane are most commonly used for inhalation induction because they have less airway irritation, none pungent odour and smooth, relatively rapid induction qualities. However halothane has potential for hepatic damage24 and increased incidence of arrhythmias. In addition sevoflurane has better advantage than halothane due to lower blood-gas solubility suggesting that induction may be more rapid than with halothane with a low incidence of complications during induction.15,25 But, halothane with its limitation has been used commonly than sevoflurane in developing countries like Ethiopia due to its availability and cost affordability.
In our country, Ethiopia, pressure response to insertion of supraglottic devices have not been compared to tracheal intubation and changes in vital signs following insertion have not been evaluated.
In our study, we found that LMA removal in deep anesthesia group presented with low incidence of airway-related adverse events (48.4%) than LMA removal in awake group (71%) (P>0.05). This high incidence of complications in awake group could be due to the difficulty of determining lightly anesthetized and fully awake state. Some pediatric age groups do not respond to verbal commands, so it may be attributed to removal of LMA in light anesthesia state. Similar to our findings, there are studies that did not found statistical difference between awake and deep group.8,11,13,14,18
Our result showed that upper airway obstruction was most frequently occurred complication (41.9% vs 35.5%) in awake and deep group, respectively and 12 patients in both groups developed desaturation (SPO2< 90%) (>0.05). This finding is in line with a randomized controlled trial conducted by Park et al.23 All these complications were managed by applying jaw thrust or inserting oral airway and administering manual positive pressure ventilation with 100% oxygen. Serious complications such as laryngospasm and breath holding rarely occurred in both groups; therefore, both states may be considered safe for LMA removal.
Kitching et al and Lee et al noted that coughing occurred in (51.5% vs. 7.4%), (37.1% vs. 2.9%) children of awake and anesthetized groups, respectively. These results were much higher than our results (12.9% vs. 6.5%). The higher incidence of coughing in former studies may be due to timing of LMA removal. Probably they remove LMA with inadequate or “light” depth of anesthesia state.13,22 Even though coughing is a sign of return of physiological response,12 it increases oxygen demand and inhibits the inability to take an adequate tidal breath, leading to desaturation, especially in younger children.26
In our study, the incidence of excessive salivation in awake group (19.4%) was lower compared a result by Lee et al (28.6%).22 This may be since the children in our study were premedicated with atropine. However, according to others studies, the current best evidence is inconclusive regarding whether the LMA should be removed early or late in patients undergoing general anesthesia. Some of them conclude that removal of LMA in anaesthetized state was associated with lesser complications than in awake condition13,16,17,19 where as others reported removal of LMA in awake condition better than in deep condition.16,22,23,27
Strength and Limitation of the Study
Beside homogeneity of the study participants this study has the following limitations.
The study is observational and limited to single center. Patients were not randomly allocated even though there were comparable groups. LMA removal was done by different anesthetists these might affect our result.
Conclusion
In conclusion, according to the findings of this study, we cannot recommend one method over the other to remove LMA, even though incidence of airway-related complications were high in awake group but there was no significant. All these complications in both groups were not serious and managed easily by applying jaw thrust or inserting oral airway and administering manual positive pressure ventilation with 100% oxygen. LMA can be removed either deeply anesthetized or awake state of anesthesia in children.
Recommendation
Therefore, we recommend further large scale randomized controlled trial study with large sample size.
Abbreviations
ASA, American Society of Anesthesiologist Physical Status; LMA, Laryngeal Mask Airway; Spo2, Arterial oxygen saturation; SPSS, Statistical Package for Social Sciences.
Data Sharing Statement
The collected data from Menelik II hospital for this study is available in soft copy as SPSS and in data collection tools as hard copy with correspondence author.
Ethics Approval and Consent to Participate
Ethical clearance and approval was obtained from the ethical review committee, anesthesia department, Addis Ababa University.
Permission was obtained from Menelik-II hospital to conduct the research. The study was undertaken on the basis of the parent’s wish by obtaining informed oral consent. There was no coercion, and or no incentives to be involved in the study. At last, the confidentiality of information obtained was secured or assured.
Acknowledgment
The authors would like to acknowledge Mr Wubshet Ayele for his contribution from inception to accomplishment of this thesis work, Addis Ababa University, Menelik II hospital, the supervisors, data collectors, and study participants for their invaluable support. Special gratitude also goes to Mr Desalegn Getnet Demsie (Msc in Pharmacology), Lecturer at Adigrat University, for his immense support.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was self-sponsored.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Miller RD, editor. Miller’s Anesthesia.
2. Van Zundert TCRV, Marcus AE. Improvements Towards Safer Extraglottic Airway Devices. Maastricht, The Netherlands: Universitaire Pers Maastricht. 2015.
3. Artime C, Hagberg CA. Airway Management in the Adult. Miler’s Anaesth.
4. Lopez-Gil M, Brimacombe J, Alvarez M. Safety and efficacy of the laryngeal mask airway. A prospective survey of 1400 children. Anaesthesia. 2020;51(10):969–972.
5. Orliaguet GA, Gall O, Savoldelli GL, Couloigner V, Riou B. Case scenario: perianesthetic management of laryngospasm in children. J Am Soc Anesthesiol. 2012;116(2):458–471.
6. Burgoyne LL, Anghelescu DL. Intervention steps for treating laryngospasm in pediatric patients. Pediatr Anesth. 2008;18(4):297–302.
7. Asai T, Koga K, Vaughan RS. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth. 1998;80(6):767–775.
8. Splinter WM, Reid CW. Removal of the laryngeal mask airway in children: deep anesthesia versus awake. J Clin Anesth. 1997;9(1):4–7.
9. Gavel G, Walker RW. Laryngospasm in anaesthesia. Contin Educ Anaesth Crit Care Pain. 2014;14(2):47–51.
10. Mamie C, Habre W, Delhumeau C, Barazzone Argiroffo C, Morabia A. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Pediatr Anesth. 2004;14(3):218–224.
11. Pappas AL, Sukhani R, Lurie J, Pawlowski J, Sawicki K, Corsino A. Severity of airway hyperreactivity associated with laryngeal mask airway removal: correlation with volatile anesthetic choice and depth of anesthesia. J Clin Anesth. 2001;13(7):498–503.
12. Nunez J, Hughes J, Wareham K, Asai T. Timing of removal of the laryngeal mask airway. Anaesthesia. 1998;53(2):126–130.
13. Kitching AJ, Walpole AR, Blogg CE. Removal of the laryngeal mask airway in children: anaesthetized compared with awake. Br J Anaesth. 1996;76(6):874–876.
14. Samarkandi AH. Awake removal of the laryngeal mask airway is safe in paediatric patients. Can J Anaesth. 1998;45(2):150–152.
15. Shim YH, Shin CS, Chang CH, Shin Y-S. Optimal end-tidal sevoflurane concentration for the removal of the laryngeal mask airway in anesthetized adults: anesth analg [internet]. Anesthesia Analgesia. 2005;101(4):1034–1037.
16. Gataure PS, Latto IP, Rust S. Complications associated with removal of the laryngeal mask airway: a comparison of removal in deeply anaesthetised versus awake patients. Can J Anaesth. 1995;42(12):1113–1116.
17. Ayele W. Comparison of awake versus deep removal of laryngeal mask airway in children aged 2 to 8 years who underwent ophthalmic procedures at menilik II hospital: cohort study. 2018.
18. Heidari SM, Abbasi S, Rahimi M. Removal of laryngeal mask airway: awake or deep anesthesia? J Res Med Sci. 2005;4.
19. NPO Guidelines - Pediatric Anesthesia Digital Handbook (maskinduction.com). Available from: https://www.maskinduction.com/npo-guidelines.html. Accessed: February 16, 2021.
20. WMA Declaration of Helsinki – ethical principles for medical research involving human subjects. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed: February 16, 2021.
21. Templeton TW, Goenaga-Díaz EJ, Downard MG, et al. Assessment of common criteria for awake extubation in infants and young children. Anesthesiology. 2019;131(4):801–808.
22. Lee J, Kim J, Kim S, Kim C, Yoon T, Kim H. Removal of the laryngeal tube in children: anaesthetized compared with awake. Br J Anaesth. 2007;98(6):802–805.
23. Park J-S, Kim K-J, Oh J-T, Choi E-K, Lee J-R. A randomized controlled trial comparing Laryngeal Mask Airway removal during adequate anesthesia and after awakening in children aged 2 to 6 years. J Clin Anesth. 2012;24(7):537–541.
24. Kenna JG, Neuberger J, Mieli-Vergani G, Mowat AP, Williams R. Halothane hepatitis in children. Br Med J Clin Res Ed. 1987;294(6581):1209–1211.
25. Sigston P, Jenkins A, Jackson E, Sury M, Mackersie A, Hatch D. Rapid inhalation induction in children: 8% sevoflurane compared with 5% halothane. Br J Anaesth. 1997;78(4):362–365.
26. Patel B, Bingham R. Laryngeal mask airway and other supraglottic airway devices in paediatric practice. Contin Educ Anaesth Crit Care Pain. 2009;9(1):6–9.
27. Huang R-C, Hung N-K, Lu C-H, Wu Z-F. Removal of laryngeal mask airway in adults under target-controlled, propofol–fentanyl infusion anesthesia: awake or deep anesthesia? Medicine (Baltimore). 2016;95(17):e3441.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.