Back to Journals » Cancer Management and Research » Volume 12
A Clinical Analysis of the Diagnosis and Treatment of Fetal Sacrococcygeal Teratomas
Authors Zheng XQ, Yan JY , Xu RL, Wang XC, Chen X, Huang KH
Received 19 October 2020
Accepted for publication 4 December 2020
Published 23 December 2020 Volume 2020:12 Pages 13185—13193
DOI https://doi.org/10.2147/CMAR.S287682
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sanjeev K. Srivastava
Xiu-Qiong Zheng,1,* Jian-Ying Yan,1 Rong-Li Xu,1,* Xue-Chun Wang,1 Xian Chen,2 Ke-Hua Huang1
1Department of Obstetrics, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou 350001, People’s Republic of China; 2Department of Obstetrics, Fujian Obstetrics and Gynecology Hospital, Fuzhou 350000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian-Ying Yan; Rong-Li Xu
Department of Obstetrics, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, No. 18, Daoshan Road, Gulou District, Fuzhou 350001, People’s Republic of China
Tel +86 591 87505886
Email [email protected]; [email protected]
Objective: The present study aims to discuss the clinical features, treatment, and prognosis of fetal sacrococcygeal teratomas (SCTs) to improve the standard of diagnosis and treatment.
Methods: The clinical data of 15 pregnant females with fetal SCT, admitted to Fujian Maternity and Child Health Hospital from January 2013 to January 2020, were retrospectively analyzed with respect to clinical characteristics, imaging features, complications, treatment options, and pregnancy outcomes.
Results: The 15 cases of fetal SCT were all detected by color ultrasonography. There were two cases of cystic tumors and 13 cases of solid cystic tumors. In terms of tumor blood supply, there was one case without blood flow signal, eight cases with little blood flow signal, and six cases with abundant blood flow. At the time of delivery, there were two cases with a tumor diameter less than 5 cm, five cases with a diameter of 5− 10 cm, and eight cases with a diameter of more than 10 cm. In terms of tumor shape and location, there were two cases of type I, ten cases of type II, and three cases of type III. There were six cases with an increased fetal heart to chest ratio, four cases of fetal edema, three cases of placental edema, four cases of excessive amniotic fluid, one case of insufficient amniotic fluid, three cases of fetal distress, one case of stillbirth, two cases of gestational diabetes mellitus, two cases of mirror syndrome, and two cases of postpartum hemorrhage. According to the pathological diagnosis, there were seven cases of mature teratoma, seven cases of immature teratoma, and one case of mixed germ cell tumor. There were six cases of induced delivery, nine cases of cesarean section, one case of premature birth, and two cases of mild neonatal asphyxia.
Conclusion: Fetal SCT was generally diagnosed by prenatal ultrasonography. The tumor blood supply, growth rate, size, nature of the tumor, clinical type, pathology, and maternal-fetal complications are all closely correlated with the prognosis. The timing and manner of the termination of pregnancy should be determined on the basis of the pregnant female, the fetus, and the tumor.
Keywords: fetus, sacrococcygeal, teratoma, therapy, prognosis
Introduction
Fetal sacrococcygeal teratoma (SCT) originates from the progenitor node or Hensen’s node of the embryonic progenitor strip. When the progenitor node persists due to developmental disorders, an SCT is formed in the sacrococcygeal region. SCT is one of the most common congenital tumors in the fetus, with an incidence of approximately 1/40,000−1/23,000, and the incidence is four times more common in females than in males.1 Fetal SCT is usually detected by prenatal ultrasonography and diagnosed with the assistance of magnetic resonance imaging (MRI). As the pregnancy progresses, there may be fetal heart failure, fetal edema, intrauterine death, excessive amniotic fluid, mirror syndrome, and other complications closely correlated with the prognosis. Therefore, early prenatal diagnosis, close supervision during the pregnancy, and close multidisciplinary cooperation are of vital importance. In the present study, the clinical data of 15 pregnant females with fetal SCT, admitted to Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, were retrospectively analyzed. The clinical characteristics, imaging features, complications, treatment options, and pregnancy outcomes were collated. The aim of the present study was to raise the awareness of fetal SCT for early detection, early monitoring, early intervention, and early treatment to improve the prognosis.
Materials and Methods
General Materials
From January 2013 to January 2020, a total of 114,376 pregnant females gave birth or had abortions in Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University. During this period, there were 15 cases of fetal SCT. The 15 pregnant women were aged between 22 to 34 years, with an average of 26.8 years. There were nine primiparas and six multiparas, none of whom were aware of having had medical diseases such as hypertension, heart disease, hepatitis, nephritis, hyperthyroidism, or a history of adverse birth and genetic diseases.
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University and informed consent was taken from all the patients.
Methods
The Diagnostic Criteria
The SCTs were classified into four types according to the tumor’s location and the extent of tumor extension into the abdominal cavity:1 Type Ⅰ: The tumor mainly protruded outside the body cavity, with only a small portion located in front of the sacrum. Type Ⅱ: The tumor protruded significantly outside the body cavity but also grew and extended into the pelvic cavity. Type III: A small portion of the tumor body protruded outside the body cavity while the main part was in the pelvic and abdominal cavity. Type Ⅳ: The tumor was only located at the anterior part of the sacrum and did not protrude outside the body cavity. Mirror syndrome: the existence of maternal edema, hemoconcentration, hypertension, proteinuria, and other pre-eclampsia-like symptoms were all secondary to fetal or placental edema of various etiologies.2
The Observation Indicators
The medical history, clinical presentation, laboratory tests, imaging data, genetic diagnosis, complications during pregnancy, timing and manner of termination of pregnancy, perinatal birth outcome, postnatal intervention, and prognosis of the neonates were collected and documented in detail. The image data were re-read and graded according to the above diagnostic classification criteria.
Results
The Clinical Characteristics of Fetal SCT
Nine cases were found by color ultrasonography screening for deformity at 22−24 weeks of gestation, and one case was found by color ultrasonography before delivery. Another five cases were found at 24–29 weeks of gestation. MRI was performed in four cases and confirmed there were no other combined abnormalities. The NT examination showed no abnormality in all 15 cases. There was one case of critical risk for trisomy 21 in early pregnancy, three cases of high risk for neural tube defects (NTD), and four cases of no abnormality detected by amniotic fluid chromosomal examination. Amniocentesis was conducted in one case with alpha-fetoprotein (AFP) greater than 2000 u/mL with the tumor pathology of mature teratoma. The examination of prenatal infection markers revealed seven cases with previous cytomegalovirus infections and six cases with previous rubella virus infections.
The Ultrasonography Features of Fetal SCT
The 15 cases were classified according to the nature and blood supply of the tumor. There were two cystic tumors: one case with no blood flow signal and one with little blood flow signal. There were 13 cases with solid cystic tumors, of which seven cases had a little blood flow signal and six cases an abundant blood flow signal. The classifications according to the tumor size at delivery were as follows: two cases with a tumor diameter of less than 5 cm, five cases with a diameter of 5−10 cm, and eight cases with a diameter of more than 10 cm. The classification based on the location of the tumor and the protruding extension of the tumor to the abdominal cavity were as follows: two cases of type I, ten cases of type II, three cases of type III, and there were no cases of type IV (as shown in Table 1, Figure 1).
 |
Table 1 The Color Ultrasonography Features and Postoperative Pathology of Fetal Sacrococcygeal Teratoma |
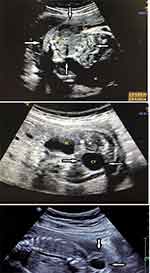 |
Figure 1 The ultrasonographic pictures of solid and cystic tumors. |
The Complications During Pregnancy
There were six cases with an increased fetal heart to chest ratio, four cases with fetal edema, three cases with placental edema, four cases with excessive amniotic fluid, one case with insufficient amniotic fluid, three cases with fetal distress, one case of stillbirth, two cases with gestational diabetes mellitus, two cases with mirror syndrome, and two cases with postpartum hemorrhage (as shown in Table 2).
 |
Table 2 The Maternal and Fetal Complications in Fetal Sacrococcygeal Teratoma |
The Timing and Manner of Terminating a Pregnancy
Four cases were given amniotic injections of Levanon at the mid-pregnancy point to induce the termination of the pregnancy. Blood pressure control and supplementation of albumin and diuretics were carried out in two cases with the occurrence of mirror syndrome. Levanon-induced labor was performed in one pregnant female at the pregnancy age of 28+5 weeks because of the aggravation of the disease. Labor was induced in one case at 30 weeks due to stillbirth and excessive amniotic fluid with a high rupture of membranes and feticide resulting from a large sacrococcygeal mass. There were nine cases of cesarean section and one case of premature birth (as shown in Table 2).
The Tumor Size/Fetal Weight Ratio (TFR)
TFR is a meaningful indicator for the early screening of fetuses at high risk of SCT and should be evaluated in conjunction with the proportion of solid cystic tumor.3 Among the 15 cases, the TFR in nine cases ranged from 0.00 to 0.42, and the TFR in one stillbirth reached as high as 0.49.
The Results of Tumor Pathology
There were seven cases of mature cystic teratoma, seven cases of immature cystic teratoma, and one case of mixed germ cell tumor (as shown in Table 1).
The Outcome and Follow-Up of Pregnancy
(1) The neonates: nine newborns were operated on in the department of pediatric surgery to remove the sacrococcygeal tumor that had been monitored up to that point, with one case having a second operation due to the reoccurrence of the tumor. There were normal urination and defecation in all the pediatric patients and no abnormalities in walking. (2) Maternity: 15 cases had a good maternal outcome. In two cases with mirror syndrome, the edema subsided, and blood pressure returned to normal after the end of the pregnancy. Re-examination 42 days after the termination revealed normal blood pressure and negative urine protein, indicating that both the anemia and hypoproteinemia were corrected.
Discussion
The Etiology of Fetal SCT
In the present study, all 15 patients were females of childbearing age. None of the fetuses had any other systemic malformations, and no abnormalities were found on the amniotic fluid chromosome and virological examination. Literature has reported that the incidence of SCT combined with chromosomal abnormalities is no higher than in the healthy population, and the cases of combined chromosomal abnormalities have other malformations, such as genitourinary or cardiovascular malformations.4 The literature also reports cases of coexistence of maternal-fetal teratomas,5 but the specific mechanism is unclear. Therefore, genetic possibilities should be considered. A detailed medical history should be taken during the prenatal examination to assess the risk factors, and chromosomal examination should be recommended if other malformations are present.
The Examinations of Fetal SCT
In the present study, there was a lack of specific clinical symptoms in the early and mid-pregnancy period. Nine cases were diagnosed by color ultrasonography screening for deformity, while one case was diagnosed by color ultrasonography before delivery. An MRI was used in four cases for further verification. Prenatal ultrasonography is the best method for the early detection and diagnosis of fetal SCT and is the primary means of screening.6 It can show the location, shape, size, blood supply, internal echo, and relationship with the surrounding tissues of the fetus in SCT, as well as indicating the presence of fetal edema, cardiac dysfunction, placental thickening, and abnormal amniotic fluid volume. There are, nevertheless, some shortcomings in ultrasonography, such as a weak soft tissue echo contrast and poor visual field. These are influenced by the operator’s technique, the thickness of abdominal fatty tissue, the fetal position, and the volume of amniotic fluid. MRI may compensate for some of the deficiencies of ultrasonography by providing better visualization of tissue density and a large window of view that is unaffected by the fetal position or insufficient amniotic fluid. It may also provide a superior resolution of the tumor tissue and the relationship to surrounding tissues.7 Therefore, for cases with an unclear ultrasonography diagnosis or unclear relationship between the tumor and surrounding tissues, the combination of ultrasonography and MRI is recommended.
The Clinical Manifestations of Fetal SCT and the Correlation with Prognosis
The Correlation Between Gestational Age at Diagnosis and Prognosis
Fourteen cases in the present study were found at 20−28 weeks of gestational age by color ultrasonography, nine of which were found by systemic color ultrasonography at 22−24 weeks, and one case was found by color ultrasonography before delivery. Teratomas have been found as early as 13+ weeks of gestation but are usually found by ultrasonography screening for deformity at 20−24 weeks of gestation. Zhong et al found that fetal mortality correlates with the gestational week of diagnosis, and the earlier the week of the initial diagnosis, the higher the mortality.4 But some research indicated the size, solid component, and degree of vascularity of fetal SCT are much more correlated with prognosis than gestational age at diagnosis. Therefore, standardized prenatal examination, prenatal consultation, and prenatal diagnosis are important prerequisites for improving the prognosis of neonates with SCT.
The Correlation Between the Blood Supply, Growth Rate, Tumor Size, Complications, and Prognosis of Fetal SCT
In the present study, nine cases of fetal SCT had little or no blood supply, with the tumor’s slow growth. There was no obvious maternal and fetal clinical manifestation. Six cases had an abundant blood supply in the tumor, with rapid growth and more complications. The incidence of complications in patients with the maximum tumor diameter higher than 10 cm was higher than in those with a diameter of less than 10 cm. The most common complications in the present study were the increased fetal heart to chest ratio, fetal edema, and excessive amniotic fluid. In addition, the richer the blood supply and the faster the growth rate of the SCT, the higher the risk of maternal and fetal complications and a poor fetal outcome. This was consistent with the findings of Chinese researchers.6 Due to the abundant blood supply and rapid growth of the fetal SCT, the tumor can shift part of the blood volume from the total fetal blood volume. This can result in complications such as fetal heart failure with high cardiac output, fetal anemia, fetal edema, placental edema, excessive amniotic fluid, preterm delivery, fetal distress, stillbirth, and maternal mirror syndrome.4 In some pregnant females, excessive weight gain, a rapid increase in uterine height and abdominal circumference, edema, and elevated blood pressure may be due to the hypoxia of the chorionic trophoblast cells, resulting in the release of anti-angiogenic factors into the maternal plasma. This may cause endothelial cell dysfunction and maternal edema, resulting in the symptoms of preeclampsia. The tumor blood supply, growth rate, and size are risk factors for the fetal prognosis.8 Large, rapidly growing SCTs with abundant blood vessels were found to have a higher incidence of perinatal mortality and complications than small SCTs with only a few blood vessels.6,9 The rapid growth rates of more than 150 cubic centimeters per week10 are associated with perinatal mortality. In the present study, in the case with rapid tumor growth, the risk of an increased fetal heart to chest ratio was higher than in the other cases. In the case with a growth rate as high as 581.79 cm3/W, the color ultrasonography repeatedly indicated abnormal fetal umbilical cord blood flow. This abnormality led to fetal edema and other adverse complications and, eventually, to induced labor. Two factors predicting stillbirth in SCT are fetal edema and prematurity.11 Fetal edema can develop from the tumor hemorrhage or arteriovenous tumor shunts leading to anemia and heart failure with high cardiac output. Therefore, fetal edema indicates a poor fetal prognosis. The diameter of inferior vena cava vessels and intravenous catheterization blood flow spectra, cardiac output, and blood flow in the middle arteries of the fetal brain are often important indicators for ultrasonography measurements. Therefore, with a definite diagnosis, the interval between the prenatal examinations should be shortened, and an ultrasonography examination should be performed regularly to monitor the blood supply, growth rate, size of the tumor, the condition of the fetus, placenta and amniotic fluid, and to evaluate the prognosis. The timing of any termination of pregnancy should be decided with full consultation with the pregnant female and her family. In the present study, a 30-week stillbirth occurred due to maternal mirror syndrome, fetal edema, increased fetal heart to chest ratio, excessive amniotic fluid, and abnormal blood flow spectrum on the pre-delivery ultrasonography. These complications led to induced labor with a high rupture of membranes. This study further suggests that the detection of multiple complications of the fetal SCT was often indicative of a poor fetal outcome. Therefore, prompt termination of pregnancy should be recommended for the prenatal ultrasonography diagnosis of an SCT with abundant blood supply, a growth rate greater than 150 cm3/week, fetal edema, chromosomal abnormalities, or other concomitant malformations.
The Correlation Between the Nature, Pathological Type, and Prognosis of Fetal SCT
Due to the complicated composition of the teratoma, ultrasonography results are diverse and can manifest as cystic, solid cystic, and solid mass images. The solid cystic tumors dominated in the present study, with just two cases of cystic tumors, similar to the findings of Feng et al.6 In the present study, the solid cystic tumors with abundant blood supply and rapid growth were mostly immature teratomas, while the cystic tumors were all mature teratomas. Studies reveal a good prognosis for cystic tumors and a poor prognosis for solid ones.9 In the case of solid tumors, several studies have demonstrated the prognostic value of the solid tumor volume index (tumor volume/estimated fetal body mass). There is a 17-fold higher risk of fetal edema when the value is >0.16.10 When the gestational age is less than 24 weeks, a TFR > 0.095 is a predictor of the adverse fetal outcome, and a TFR > 0.12 is a predictor of an increased risk of maternal surgery. TFR > 0.11 before the gestational age of 32 weeks predicts a poor fetal prognosis.12 Wohlmuth13 found that tumor size to fetal weight ratio has been found to be unreliable, but these studies have shown that the tumor’s vascularization plays a critical role in terms of prognosis. However, in the present study, the TFR ranged between 0.00 and 0.53 at the time of delivery, with the TFR in nine neonates fluctuating between 0.00 and 0.42 and the TFR in one case of stillbirth reaching as high as 0.49. All nine pregnant females were delivered by cesarean section, and the higher the TFR, the higher the risk of associated complications for the mother and fetus. Some researchers found that when the fetal tumor/biparietal diameter ratio was greater than 60 cm2,14 the postnatal survival was low. It was also reported that if the tumor was an immature teratoma containing yolk sac or embryonic components, it could secrete AFP into the blood, leading to an increase in maternal serum AFP. Thus, the AFP in the maternal blood could be used as an auxiliary index for the non-invasive prenatal evaluation of fetal SCT.15 There was only one case of a mature teratoma undergoing amniocentesis with an AFP greater than 2000 u/mL, and AFP was not detected in the remaining cases. In the present study, nine neonates were operated on in the department of pediatric surgery to remove the SCT, and one case was operated on twice for tumor recurrence after surgery. A clear trend of a decrease in AFP was observed during the regular postoperative follow-up. Therefore, further studies are needed to determine whether high maternal serum and amniotic fluid AFP are associated with poor fetal outcomes.
The Correlation Between Clinical Type and Prognosis
In the present study, fetal edema and cardiac dysfunction were present in all cases of type III, while there were fewer maternal and fetal complications in cases of type I and type II. This suggests that the clinical typing of the tumor correlates with the prognosis, and the prognosis was poor in the fetus with type III. A study by Shue et al13 found that the prognosis of pediatric patients with type III and IV was poorer than that of patients with type I and II. It was postulated that the mode in which the tumor grows might correlate with the difficulty of the surgical approach. The surgery for types I−IV ranges from easy to difficult, and the possibility of malignancy ranges from low to high.
The Timing and Manner of Delivery of Fetal SCT
The timing and manner of delivery of fetuses with SCT are determined by the maternal-fetal complications, tumor size, growth pattern, fetal orientation, and maternal birth canal conditions, and many other factors. In the present study, an abortion was induced in two cases with mirror syndrome, one of which had excessive amniotic fluid. The stillbirth was induced by the high rupture of membranes and feticide to avoid injury to the birth canal. Labor was induced by Levanon in one case. Nine neonates survived the prenatal period, with one case of premature birth due to fetal distress and eight cases of a full-term birth. All the neonates had to be delivered by cesarean section due to the large size of the fetal sacral tumor to avoid difficult delivery or tumor rupture. Baumgarten’s study found that the gestational week at delivery was also an important factor for the prognosis.16 Therefore, we recommend early termination of pregnancy for SCTs that are considered to be immature teratomas or tumors with rapid growth rates, or SCT at specific locations that might affect fetal growth. For those considered to be mature teratomas with slow growth and little effect on fetal development, the condition should be monitored closely. If there is no obvious maternal and fetal abnormality, the fetus could be delivered at full term. In the case of fetal edema, fetal cardiac dysfunction, or maternal mirror syndrome, the pregnancy should be terminated as early as possible. Literature has recommended that for tumors larger than 5 cm in diameter, a cesarean section is recommended for a completion of pregnancy.17 Vaginal labor is preferred for cases with tumors less than 5 cm in diameter provided the maternal and fetal situations are good.6
The Management of a Multidisciplinary Team in Fetal SCT
Fetal SCT has a low incidence, and treatment is still elusive, so there are no guidelines or expert consensus to follow. Currently, treatment is mainly individualized, based on the maternal and fetal condition and the patient’s wishes. This includes symptomatic treatment, fetal intrauterine treatment, termination of pregnancy, and treatment for neonates. Fetal intrauterine therapy might be considered when the fetus has cardiac abnormalities and signs of fetal edema before reaching the viable gestational age. The aim of intrauterine therapy, such as vascular embolization, radio-frequency ablation, alcohol sclerosis, and open fetal surgery,9,18 is to prevent arterial-venous shunting and reduce the tumor size or remove the tumor completely. However, it can result in complications such as preterm delivery, intrauterine stillbirth, and maternal injury. The current medical standard of fetal intrauterine treatment is limited, and successful cases are still rare. Therefore, it is necessary to communicate clearly with pregnant females with a diagnosis of fetal SCT, as well as with their families, to inform them of all the possible adverse effects. Moreover, joint multidisciplinary diagnosis and treatment can improve the prognosis of the fetus.19 In the present study, a multidisciplinary team was organized for consultation before a cesarean delivery in one case of fetal cardiac insufficiency, fetal distress, breech position, and a large sacrococcygeal tumor (18×16×12 cm3). The team consisted of neonatology, pediatric surgery, anesthesiology, ultrasonography, and medical imaging clinicians, thus ensuring the effective treatment of the fetus. In order to achieve the smooth delivery of the baby and avoid rupture of the tumor, the incision was designed as a vertical incision of the upper abdominal wall and an inverted T-shaped incision for the uterus. The pregnant female presented with excessive amniotic fluid and overexpansion of the uterus. Therefore, intraoperative active use of a variety of means of hemostasis, such as strong vasoconstrictors, uterine binding sutures, and ligation of the upper uterine artery, was done to prevent postpartum hemorrhage. After delivery, a neonatologist resuscitated the neonate. After the vital signs stabilized, a pediatric surgeon operated on the neonate, giving a good prognosis.
The Intervention of Fetal SCT After Birth
The majority of SCTs are reportedly benign during the fetal and neonatal period, with 20% malignancy within two months after birth and up to 40% after four months.4 Therefore, complete removal should be performed as soon as possible after birth. In the present study, all the cases had been surgically treated for SCT within one week of birth, and no intestinal or urinary tract dysfunction had been observed during the follow-up. Among them, one case of mature teratoma recurred after surgery, and the patient underwent surgery again. No related intestinal and urinary system dysfunction has been observed so far. Both mature and immature teratomas have the same potential for malignancy and recurrence8 and require regular follow-up.
Conclusion
In summary, fetal SCT is a rare disease, but it can cause harm to both maternal and fetal health. The gestational age at the time of initial diagnosis, tumor blood supply, growth rate, tumor size, tumor nature, pathology, clinical type, and maternal-fetal complications are all closely correlated with the prognosis. With the prenatal diagnosis of fetal SCT, adequate communication should be facilitated with the pregnant female and her family to understand the development, evolution, diagnosis, treatment, and prognosis of the disease fully. Close monitoring of the tumor growth, fetal growth, fetal heart function, edema, amniotic fluid volume, and the maternal condition is required. The timing and manner of termination of pregnancy should be determined according to the condition of the pregnant female, the fetus, and the tumor. Perioperative multidisciplinary management can be effective in improving neonatal outcomes.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
This study was funded by:1. Guide Fund for the Development of Local Science and Technology from the Central Government (2020L3019)2. National Health and Family Planning Commission Science Foundation (2019-WJ-04)3. Fujian Science and Technology Project (2018Y0005)4. Key Clinical Specialty Discipline Construction of Fujian, P.R.C ([2015] no. 593)The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Li SL, Luo GY. Fetal Malformations Prenatal Ultrasonography.
2. Braun T, Brauer M, Fuchs I, et al. Mirror syndrome: a systematic review of fetal associated conditions, maternal presentation and perinatal outcome. Fetal Diagn Ther. 2010;27(4):191–203. doi:10.1159/000305096
3. Gebb JS, Khalek N, Qamar H, et al. High tumor volume to fetal weight ratio is associated with worse fetal outcomes and increased maternal risk in fetuses with sacrococcygeal teratoma. Fetal Diagn Ther. 2019;45(2):94–101. doi:10.1159/000486782
4. Zhong L, Wang XD, Yu HY. Advances in fetal sacral teratoma research. J Pract Obstet Gynecol. 2019;35(1):24–26.
5. Guan H, Lu CM, Shang LX. Co-existence of maternal mature ovarian teratoma and fetal immature sacrococcygeal teratoma: a case report and literatures review. J Dev Med. 2018;6(03):186–189.
6. Feng X, Liu XJ, Ma QP. Application of prenatal ultrasound in diagnosis and treatment of fetal sacrococcygeal teratoma Chinese. J Prenat Diagn. 2020;12(01):20–23.
7. Zhang L, Chen X, Yang XH, et al. Combined application of prenatal ultrasound and magnetic resonance imaging in diagnosis of fetal teratoma. Chin J Ultrasound Med. 2017;33(10):923–925.
8. Fumino S, Tajiri T, Usui N, et al. Japanese clinical practice guidelines for sacrococcygeal teratoma, 2017. Pediatr Int. 2019;61(7):672–678. doi:10.1111/ped.13844
9. Litwińska M, Litwińska E, Janiak K, Piaseczna-Piotrowska A, Szaflik K. Percutaneous intratumor laser ablation for fetal sacrococcygeal teratoma. Fetal Diagn Ther. 2020;47(2):138–144. doi:10.1159/000500775
10. Coleman A, Shaaban A, Keswani S, Lim FY. Sacrococcygeal teratoma growth rate predicts adverse outcomes. J Pediatr Surg. 2014;49(6):985–989. doi:10.1016/j.jpedsurg.2014.01.036
11. Ayed A, Tonks AM, Lander A, Kilby MD. A review of pregnancies complicated by congenital sacrococcygeal teratoma in the West Midlands region over an 18-year period: population-based, cohort study. Prenat Diagn. 2015;35(11):1037–1047. doi:10.1002/pd.4641
12. Baumgarten HD, Gebb JS, Khalek N, et al. Preemptive Delivery and Immediate Resection for Fetuses with High-Risk Sacrococcygeal Teratomas. Fetal Diagn Ther. 2019;45(3):137–144. doi:10.1159/000487542
13. Wohlmuth C, Bergh E, Bell C, Johnson A, Moise KJ Jr, van Gemert MJC, van den Wijngaard JPHM, WohlmuthWieser I, Averiss I, Gardiner HM. Clinical monitoring of sacrococcygeal teratoma. Fetal Diagn Ther. 2019;46(5):333–340. doi:10.1159/000496841
14. Lee SM, Suh DH, Kim SY, et al. Antenatal prediction of neonatal survival in sacrococcygeal teratoma. J Ultrasound Med. 2018;37(8):2003–2009. doi:10.1002/jum.14553
15. Goto S, Suzumori N, Obayashi S, Ozaki Y, Sugiura-Ogasawara M. Two cases of prenatally diagnosed sacrococcygeal teratoma type I with different clinical features. Congenit Anom (Kyoto). 2013;53(2):92–94. doi:10.1111/j.1741-4520.2012.00369.x
16. Baumgarten HD, Gebb JS, Khalek N, et al. Preemptive delivery and immediate resection for fetuses with high-risk sacrococcygeal teratomas. Fetal Diagn Ther. 2019;45(3):137–144. doi:10.1159/000487542
17. den Otter SC, de Mol AC, Eggink AJ, van Heijst AF, de Bruijn D, Wijnen RM. Major sacrococcygeal teratoma in an extreme premature infant: a multidisciplinary approach. Fetal Diagn Ther. 2008;23(1):41–45. doi:10.1159/000109225
18. Stavropoulou D, Hentschel R, Rädecke J, et al. Preoperative selective embolization with vascular coiling of giant sacrococcygeal teratoma. J Neonatal Perinatal Med. 2019;12(3):345–349. doi:10.3233/NPM-180066
19. Liang JP, LiI XZ, Yang Z. A case of large fetal sacrococcygeal teratoma. Chin J Obstet Gynecol. 2019;54(3):196–197.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
