Back to Archived Journals » Oncolytic Virotherapy » Volume 8
A cautionary note on the selectivity of oncolytic poxviruses
Authors Tang B, Guo ZS , Bartlett DL, Liu J , McFadden G , Shisler JL, Roy EJ
Received 5 October 2018
Accepted for publication 31 December 2018
Published 11 February 2019 Volume 2019:8 Pages 3—8
DOI https://doi.org/10.2147/OV.S189832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Faris Farassati
Bingtao Tang,1 Zong Sheng Guo,2 David L Bartlett,2 Jia Liu,3 Grant McFadden,4 Joanna L Shisler,5 Edward J Roy1
1Department of Molecular and Integrative Physiology, University of Illinois Urbana-Champaign, Urbana, IL, USA; 2Department of Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; 3Department of Microbiology and Immunology, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 4Biodesign Institute, Arizona State University, Tempe, AZ, USA; 5Department of Microbiology, University of Illinois Urbana-Champaign, Urbana, IL USA
Background: Oncolytic viruses selectively infect cancer cells while avoiding infection of normal cells. Usually, selectivity is demonstrated by injecting a virus into tumor-bearing mice and observing infection and lysis of tumor cells without infection of other tissues. The general view is that this selectivity is due to tropisms of the virus. However, apparent selectivity could be due to accessibility. For example, intravenously injected virus may not gain access to cells within the central nervous system (CNS) because of the blood–brain barrier.
Purpose: We tested the CNS safety of two oncolytic poxviruses that have been demonstrated to be safe for treatment of peripheral tumors (vaccinia virus vvDD-IL15-Rα and myxoma virus vMyx-IL15Rα-tdTr).
Methods: Two poxviruses were tested for selectivity in vitro and in vivo.
Results: Both viruses infected glioma cells in vitro. In vivo, both viruses infected glioma cells and did not infect neurons when injected into a tumor or into the normal striatum. However, viral gene expression was observed in ependymal cells lining the ventricles, implying that these poxviruses were not as selective as originally predicted. For vvDD-IL15-Rα, some tumor-bearing mice died soon after virus treatment. If the same titer of vvDD-IL15-Rα was injected directly into the lateral cerebral ventricle of nontumor-bearing mice, it was uniformly fatal. Infection of ependymal cells, subventricular cells, and meninges was widespread. On the other hand, vMyx-IL15Rα-tdTr only transiently infected ependymal cells and was safe even when injected directly into the lateral cerebral ventricles. The two poxviruses also differed in their infection of dendritic cells; vvDD-IL15-Rα infected dendritic cells and lysed them but vMyx-IL15Rα-tdTr did not.
Conclusion: Vaccinia virus vvDD-IL15-Rα is very promising for treating cancer types outside of the brain. However, for cancers located within the brain, myxoma virus vMyx-IL15Rα-tdTr offers a safer alternative.
Keywords: oncolytic virus, central nervous system toxicity, glioma, safety
Introduction
There are a large number of oncolytic viruses that may selectively infect and lyse cancer cells while avoiding infection of normal cells.1 A variety of mechanisms contribute to the selectivity of oncolytic viruses for cancer cells over normal cells. For example, EGFR activation and altered type I interferon responses, common in cancer cells, contribute to a productive infection by the attenuated vaccinia virus JX-594.2 Normally, evidence for cancer selectivity is derived from in vivo experiments; a virus is administered intravenously or intraperitoneally, and if it infects tumor cells but does not result in widespread infection of normal tissues, it is considered cancer selective. However, this model ignores the fact that some cell types are relatively inaccessible to an oncolytic virus and may not be infected for that reason. For example, cells within the central nervous system (CNS) might be susceptible to viral infection, but do not come into contact with an oncolytic virus because of the blood–brain barrier. Therefore, oncolytic viruses that might be used to treat brain tumors need to be tested for selectivity within the CNS.
Bartlett’s laboratory developed an attenuated vaccinia virus that lacks thymidine kinase and the vaccinia growth factor (vvDD).3 vvDD has undergone two phase I clinical trials and has been found safe in humans.4,5 vvDD was subsequently engineered to carry a gene for expression of a superagonist fusion protein of IL15 and IL15Ra, and this virus was used to treat colon cancer in mice. When combined with anti-PD1 treatment, vvDD-IL15-Rα totally eradicated established colon cancer in 100% of mice.6 When we applied the same vvDD-IL15-Rα to treat intracerebral gliomas in mice (2×106 pfus), the virus was highly effective in eliminating gliomas when combined with adoptive T-cell therapy and a prostaglandin synthesis inhibitor (unpublished data). However, in each cohort there was a single mouse that died within 4–5 days after virus infusion into the tumor, well before the mice might be expected to die from tumor progression. This raised the possibility that vvDD-IL15-Rα was infecting and lysing more than just tumor cells. In the present study, we investigated the selectivity of vvDD-IL15-Rα for cancer cells vs normal tissue within the CNS.
We also investigated a related poxvirus, myxoma virus vMyx-IL15Rα-tdTr, that may be a safer alternative for treatment of tumors within the CNS. We and others previously observed that myxoma virus can be used to selectively infect and lyse glioma cells when injected directly into a tumor.7–11 There was no infection of normal brain cells when the virus was injected into the normal striatum. When injected into the lateral cerebral ventricle, viral gene expression could be observed for several days but was no longer observable after 11 days post injection, and mice showed no adverse consequences of viral exposure.12 We therefore investigated in the present study whether myxoma virus expressing the same IL15–IL15Rα fusion protein might be safer than vvDD-IL15-Rα.
Materials and methods
Mice
C57BL/6J mice of both sexes were obtained from Jackson Laboratory (Bar Harbor, ME, USA) or bred in facilities at the University of Illinois Division of Animal Resources (Urbana, IL, USA), and were between 2 and 4 months of age. Mice were maintained on a 14:10 hours light–dark cycle and singly housed after infusion of tumor cells or virus. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign, which adheres to the Animal Welfare Act and its implementation by the National Institutes for Health Office of Laboratory Animal Welfare, as described in the Guide for the Care and Use of Laboratory Animals, 8th edition. The University of Illinois at Urbana-Champaign is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Tumor cell line
GL261 was obtained from the National Cancer Institute – Frederick Cancer Research Tumor Repository (Frederick, MD, USA) and initially grown in DMEM containing 5 mM HEPES, 1.3 mM l-glutamine, 50 mM 2-mercaptoethanol, penicillin, streptomycin, and 10% FBS at 37 °C and 5% CO2. Beginning 1 week before use, GL261 cells were grown under neurosphere conditions (GL261 NS).13 They were cultured in untreated cell culture flasks (Corning Incorporated, Corning, NY, USA) with DMEM/F12+ GlutaMAX (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with penicillin/streptomycin (Lonza, Walkersville, MD, USA), B27 with vitamin A (Thermo Fisher Scientific), 20 ng/mL recombinant human EGF (R&D Systems Inc., Minneapolis, MN, USA), 20 ng/mL recombinant human fibroblast growth factor (R&D Systems Inc.), and 5 µg/mL heparin (Sigma-Aldrich Co., St. Louis, MO, USA) in an incubator at 37 °C with 5% CO2.14
Viruses
Myxoma virus vMyx-IL15Rα-tdTr contains genes for the IL15-IL15Rα fusion protein and tdTomato, both under control of the same synthetic vaccinia virus early/late promoter, as previously described.15 The generation and validation of vvDD expressing the same IL15–IL15Rα construct and yellow fluorescent protein (YFP) (vvDD-IL15-Rα) has been described previously.6 Viruses used in vivo were sucrose pad purified and adjusted to a titer of 2×109 pfu/mL. As a control for potential effects of IL15–IL15Rα, vvDD-RFP, which expresses red fluorescent protein but not IL15–IL15Rα, was also tested.
Intracerebral cancer cell and virus infusions
GL261 NS cells were harvested, washed twice with Hanks’ Balanced Salt Solution (HBSS; Mediatech, Inc., Manassas, VA, USA), and stereotaxically infused into the brains of mice anesthetized with isoflurane. 5×104 GL261 NS cells in 0.5 µl HBSS were infused into the ventral striatum (0.5 mm rostral to the bregma, 2.25 mm lateral, 3.3 mm ventral). After 7 days, tumor-bearing mice were anesthetized and stereotaxically injected intratumorally with 1 µl PBS containing either 2×106 pfu vvDD-IL15-Rα, 2×106 pfu vMyx-IL15Rα-tdTr, or no virus. For intracerebroventricular infusion of virus in nontumor-bearing mice, coordinates were 0 mm rostral to the bregma, 1.0 mm lateral, 3.0 mm ventral. Mice were monitored daily and euthanized if their body weight dropped to 75% of baseline body weight, or if there were signs of neurological impairment or lethargy, in accordance with IACUC guidelines.
Immunohistochemistry
Five-micron sections were cut by cryostat. Slides were incubated at –20 °C in 95% ethanol for 20 minutes, washed with PBS, blocked with SuperBlock (Thermo Fisher Scientific), and incubated overnight at room temperature with primary antibody, 1:100 dilution of rabbit anti-vaccinia antibody (YVS8101; Accurate Chemical and Scientific Corp, Westbury, NY, USA) in 20% glycerol/5% SuperBlock in PBS. The secondary antibody was (Fab′)2 Alexa 647 anti-rabbit (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and the counterstain was DAPI (Thermo Fisher Scientific). Six sections per brain were analyzed. On an additional set of six brains containing a GL261 tumor (three treated with vvDD-IL15-Rα and three treated with PBS), staining was done to determine whether vvDD infected microglia or macrophages. Dual staining was done with anti-vaccinia antibody YVS8101 (made in rabbit) and anti-CD11b (clone M1/70, made in rat; BioLegend, San Diego, CA, USA), using (Fab′)2 Alexa 488 anti-rabbit and (Fab′)2 Alexa 647 anti-rat secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.).
Bone marrow-derived dendritic cells
Bone marrow was harvested from the tibia and femur of adult mice, red blood cells were lysed with ammonium chloride potassium, and cells were cultured in RPMI containing 20 ng/mL granulocyte–macrophage colony-stimulating factor. After 6 days, cells were plated in 24-well plates and incubated with virus at a multiplicity of infection of 5. Cells were harvested after 24 hours, and cells were counted and analyzed for YFP (for vvDD-IL15-Rα) and tdTomato red (for vMyx-IL15Rα-tdTr) expression by flow cytometry. Cellular viability was assessed by Trypan blue dye exclusion.
Flow cytometry
Cells were analyzed on a BD Fortessa flow cytometer (BD Biosciences, San Jose, CA, USA) at the University of Illinois Biotechnology Center (Urbana, IL, USA) for viral expression of YFP (vvDD-IL15-Rα) and tdTomato red (vMyx-IL15Rα-tdTr).
Statistical analyses
ANOVAs with multiple comparisons with Bonferroni’s correction were carried out using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). Differences in fatalities between vvDD-IL15-Rα and vMyx-IL15Rα-tdTr were analyzed by Fisher’s exact test.
Results
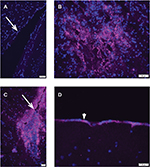
Brains from control PBS-treated mice did not exhibit staining with anti-vaccinia antibody in cells lining the lateral cerebral ventricles (Figure 1A), indicating specificity of the primary antibody for poxvirus antigens. The brains from the 3 mice (of 26 treated mice) that died shortly after infusion of vvDD-IL15-Rα virus were stained with an antibody against vaccinia antigens and examined histologically. Tumor cells stained positive for virus, as expected, suggesting that vvDD-IL15-Rα infected tumor cells (Figure 1B). Notably, in the mice that died, the virus had spread to ependymal cells of the lateral ventricle (Figure 1C) and meningeal cells on the surface of the brain (Figure 1D). Similarly strong staining for virus was widespread, observed in both lateral ventricles and the third ventricle (data not shown).
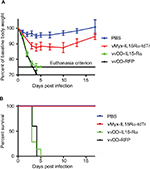
To determine whether viral infection of ependyma and meninges was responsible for these deaths, we injected the same dose of vvDD-IL15-Rα directly into the lateral cerebral ventricle of a group of nontumor-bearing mice. The results are shown in Figure 2A, B. All mice receiving vaccinia virus either died or reached the criterion for euthanasia (75% of baseline body weight) within 4–5 days. To control for the potential effects of the IL15–IL15Rα fusion protein, an additional group of mice received vvDD-RFP. Both vvDD viruses were fatal when injected intracerebroventricularly (Figure 2). In contrast, no mice that received myxoma virus expressing the same IL15–IL15Rα construct died (Figure 2) (P<0.0001). Therefore, the vvDD virus rather than the IL15–IL15Rα fusion protein was responsible for toxicity.
We also compared vvDD-IL15-Rα and vMyx-IL15Rα-tdTr infection of another noncancer cell type, dendritic cells. The rationale for this was that wild type vaccinia viruses are known to have a tropism for dendritic cells,16 while myxoma virus does not. We compared viral infection of glioma cells and dendritic cells for each virus. Both vvDD-IL15-Rα and vMyx-IL15Rα-tdTr expressed their respective fluorescent proteins in GL261 cells, analyzed by flow cytometry at 24 hours post infection (Figure 3A). Both viruses had a lytic effect on GL261 (Figure 3B). On the other hand, only vvDD-IL15-Rα clearly infected bone marrow-derived dendritic cells (Figure 3C), and by 24 hours the majority of cells had been lysed (Figure 3D). The remaining alive cells expressed fluorescent protein, as assessed by flow cytometry (Figure 3C). In contrast, vMyx-IL15Rα-tdTr did not lyse any bone marrow-derived dendritic cells and very little fluorescent protein expression was evident by flow cytometry (Figure 3C, D).
Because it has been reported that a related vaccinia virus (Lister strain LIVP 1.1.1) infects BV1 microglial cells to a low degree,17 we investigated whether vvDD-IL15-Rα infects microglia in vivo in mice bearing GL261 tumors. Brain sections were dual-stained with anti-vaccinia virus antibody and anti-CD11b (which stains both microglia and macrophages) and images were analyzed for colocalization using ImageJ (National Institutes of Health, Bethesda, MD, USA). Within the tumor there was extensive infiltration by microglia/macrophages, but very little colocalization of CD11b and vaccinia protein (colocalization coefficient m1=0.016 and m2=0.018). Outside of the tumor we observed no evidence of infection of microglia by vvDD-IL15-Rα.
Discussion
The mechanistic basis for the selectivity of oncolytic viruses for cancer cells varies with the virus. There is no convenient way to test all tissue types with a candidate oncolytic virus, other than to systemically administer the virus and determine whether infection spreads. The results reported here indicate that access to some cell types is limited and can mask the viral susceptibility of certain cell types. Specifically, ependymal and meningeal cells within the brain are susceptible to infection by attenuated vaccinia virus, and if the virus gains access to these cells the results can be fatal. This result is not completely surprising, given that the Western Reserve strain of vaccinia virus, which is the parental virus for vvDD, was selected by serial passage in the brain of mice.18 Lun et al19 reported that in rats, systemic treatment of gliomas with vvDD-EGFP was safe but direct intratumoral injection of the virus was highly toxic. Perhaps the toxicity was related to viral infection of ependymal and meningeal cells, which was not mentioned in that report. In contrast to vaccinia virus, the parental virus for vMyx-IL15Rα-tdTr, myxoma virus, has a very limited host range (European rabbits).20 We therefore suggest that vvDD-IL15-Rα is very promising for treating cancer types outside of the brain. For cancers located within the brain, myxoma virus vMyx-IL15Rα-tdTr offers a safer alternative.
Acknowledgments
This research was funded by institutional funds from the University of Illinois Urbana-Champaign. We thank the people of the Biotechnology Center Flow Cytometry Facility for cheerful and competent service. We thank Claire Schane, David Yan, and Corinne Cannavale for technical assistance.
Disclosure
ZSG and DLB serve as scientific advisors to ICell Kealex Therapeutics. DLB has financial interest with SillaJen Biotherapeutics. GM is a co-founder of OncoMyx Therapeutics, devoted to the clinical development of Oncolytic Myxoma Virus. The authors report no other conflicts of interest in this work.
References
Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. | ||
Parato KA, Breitbach CJ, Le Boeuf F, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20(4):749–758. | ||
McCart JA, Ward JM, Lee J, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61(24):8751–8757. | ||
Zeh HJ, Downs-Canner S, McCart JA, et al. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23(1):202–214. | ||
Downs-Canner S, Guo ZS, Ravindranathan R, et al. Phase 1 study of intravenous oncolytic poxvirus (vvDD) in patients with advanced solid cancers. Mol Ther. 2016;24(8):1492–1501. | ||
Kowalsky SJ, Liu Z, Feist M, et al. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol Ther. 2018:26(10):2476–2486. | ||
Lun X, Alain T, Zemp FJ, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70(2):598–608. | ||
Lun X, Yang W, Alain T, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65(21):9982–9990. | ||
Thomas DL, Doty R, Tosic V, et al. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol Immunother. 2011;60(10):1461–1472. | ||
Zemp FJ, Lun X, McKenzie BA, et al. Treating brain tumor-initiating cells using a combination of myxoma virus and rapamycin. Neuro Oncol. 2013;15(7):904–920. | ||
Ogbomo H, Zemp FJ, Lun X, et al. Myxoma virus infection promotes NK lysis of malignant gliomas in vitro and in vivo. PLoS One. 2013;8(6):e66825. | ||
France MR, Thomas DL, Liu J, McFadden G, MacNeill AL, Roy EJ. Intraventricular injection of myxoma virus results in transient expression of viral protein in mouse brain ependymal and subventricular cells. J Gen Virol. 2011;92(Pt 1):195–199. | ||
Pellegatta S, Poliani PL, Corno D, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66(21):10247–10252. | ||
Pellegatta S, Finocchiaro G. Dendritic cell vaccines for cancer stem cells. Methods Mol Biol. 2009;568:233–247. | ||
Tosic V, Thomas DL, Kranz DM, et al. Myxoma virus expressing a fusion protein of interleukin-15 (IL15) and IL15 receptor alpha has enhanced antitumor activity. PLoS One. 2014;9(10):e109801. | ||
Chahroudi A, Chavan R, Kozyr N, et al. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397–10407. | ||
Kober C, Rohn S, Weibel S, Geissinger U, Chen NG, Szalay AA. Microglia and astrocytes attenuate the replication of the oncolytic vaccinia virus LIVP 1.1.1 in murine GL261 gliomas by acting as vaccinia virus traps. J Transl Med. 2015;13(1):216. | ||
Wokatsch R. Vaccinia virus. In: Majer M, Plotkin SA, editors. Strains of Human Viruses. Basel: Kerger; 1972; 241–257. Available from: https://www.karger.com/Article/FullText/393659. Accessed September 3, 2018. | ||
Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15(8):2777–2788. | ||
Mossman K, Lee SF, Barry M, Boshkov L, McFadden G. Disruption of M-T5, a novel myxoma virus gene member of poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J Virol. 1996;70(7):4394–4410. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.