Back to Journals » Infection and Drug Resistance » Volume 15
A Case Report of Herpes Zoster-Associated Bickerstaff Brainstem Encephalitis
Authors Li M , Wang X , Chen M , Chang Y , Li L , Zhong S
Received 17 May 2022
Accepted for publication 1 August 2022
Published 22 August 2022 Volume 2022:15 Pages 4759—4762
DOI https://doi.org/10.2147/IDR.S374981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Man Li,1,* Xingyu Wang,2,* Mojun Chen,1 Yuan Chang,1 Linfeng Li,1 Shan Zhong1
1Department of Dermatology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Dermatology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shan Zhong, Department of Dermatology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China, Tel +86-13810056088, Fax +86-10-63139108, Email [email protected]
Background: Bickerstaff brainstem encephalitis (BBE) is a rare demyelinating disease of the central nervous system (CNS) that is caused by a direct viral infection or secondary autoimmune responses. BBE secondary to Herpes zoster has rarely been reported.
Case Presentation: A 68-year-old man developed a painful vesicular rash and drooping eyelid on the left side of his face for 20 days. Physical examination revealed left-sided blepharoptosis and crusted erythema on the left front side of his face, left upper eyelid, and left nasal tip. Neurological examination showed impaired sensation over the left side of his face and cheek. His left pupil was dilated (4mm compared to 2mm on the right side), and the Pupillary light reflection (PLR) was absent, with an ocular movement disorder (limited adduction) and diplopia. Brain imaging did not reveal abnormalities. Cerebrospinal fluid (CSF) examination showed leukocytosis and increased protein levels. He was treated with intravenous acyclovir for 7 days, but developed disturbance of consciousness and right limb weakness. Neurological examination revealed right lower limb hypoesthesia. The Heel-Knee-Shin test was positive on the left side, and Babinski’s sign was present on the right side. He was diagnosed with Bickerstaff brainstem encephalitis caused by herpes zoster. After 2 days of intravenous acyclovir combined with intravenous immune globulin (IVIG), the patient developed acute kidney injury (AKI). Then, his treatment was changed to systemic steroids. At the 3-month follow-up, his pupils were bilaterally equal and reactive to light, and there was a significant improvement in ocular motility and ptosis. At the 6-month follow-up, his diplopia had completely resolved.
Conclusion: BBE associated with herpes zoster is very rare and can be overlooked. Dermatologists should be aware of the expanding spectrum of neurological complications caused by varicella zoster virus (VZV) infections to aid early diagnosis and treatment.
Keywords: herpes zoster, Bickerstaff brainstem encephalitis, acute kidney injury, treatment
Introduction
Herpes zoster caused by the varicella zoster virus (VZV) is characterized by vesicular eruptions in a dermatomal distribution with unilateral radicular pain. VZV is a neurotropic DNA virus, thus herpes zoster can also present with neurological manifestations, such as meningitis, cerebellitis, encephalitis, myelitis, cerebral vasculopathies, cranial nerve inflammation, and, rarely, peripheral motor neuropathy and stroke-related syndromes.1,2 Bickerstaff’s brainstem encephalitis (BBE) is a rare syndrome defined by the triad of ophthalmoplegia, ataxia, and decreased consciousness.3 A Japanese survey estimated the annual incidence of BBE to 0.078/100 000, with an annual onset of 100 cases.4 The etiology and pathogenesis of BBE are unknown and may be related to viral infections (such as herpes simplex and Epstein-Barr virus) or autoimmune demyelination. However, BBE subsequent to Herpes zoster has rarely been reported. Here, we describe a case of successfully treated herpes zoster-associated BBE.
Case Presentation
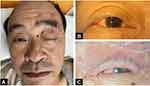
A 68-year-old man developed a painful vesicular rash followed by a drooping eyelid for 20 days on the left side of his face. He was diagnosed with herpes zoster and conjunctivitis in local hospital settings and treated with 600mg of oral valaciclovir every day for 2 weeks. The vesicles started to heal, but the pain and left-sided blepharoptosis persisted. He had a 20-year history of hypertension, type 2 diabetes, and coronary artery disease (CAD). Physical examination revealed left-sided blepharoptosis, crusted erythema on the left side of his face, left upper eyelid, and nasal tip (Figure 1A). Neurological examination showed impaired sensation over the left side of the face and left cheek. His left pupil was dilated (4mm vs 2mm for the right) and both the direct and indirect Pupillary light reactions (PLR) were absent, with an ocular movement disorder (limited adduction) and diplopia, all of which indicated complete injury of the left oculomotor nerve (Figure 1B and C). No meningeal irritating signs were detected. Deep tendon reflexes were symmetric and normal, and Babinski’s sign was not observed.
Urine, hematology, and biochemical test results were normal. Computed tomography angiography (CTA), magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) showed no brainstem abnormalities. Cerebrospinal fluid (CSF) from a lumbar puncture on day 5 after admission to the hospital showed normal pressure and was clear with leukocytosis at a level of 73 × 10^6/L (normal range: 0–8 × 10^6/L). The CSF protein level was 175.50 (normal range: 15–45mg/dl), and the CSF sugar level was 4.29 (normal range: 2.24–3.92mmol/L). The CSF polymerase chain reaction (PCR) for VZV-DNA was negative.
The patient was diagnosed with polyneuritis cranialis (oculomotor nerve palsy and dysfunction of the trigeminal nerve I/II branch), most likely secondary to herpes zoster infection. Intravenous acyclovir (500mg/day) was given for 7 days. However, the patient’s symptoms were not significantly improved, and he developed disturbance of consciousness (somnolence and paraphasia) and right limb weakness. Neurologic examination revealed hypoesthesia (decreased puncture sensation) in the right lower limb. In addition, his right lower limb strength was weak (4/5), while the left lower limb and both upper limbs had normal strength (5/5). The Heel-Knee-Shin test was positive on the left side and Babinski’s sign was positive on the right side.
Taken together, the above evidence suggested that the patient had cerebellar and spinothalamic tract impairments. Bickerstaff brainstem encephalitis caused by VZV infection was suspected based on the localization and qualitative diagnosis. After 2 days’ of intravenous acyclovir (750mg bid), combined with 27.5g/day intravenous immunoglobulin therapy (IVIG), the patient developed acute kidney injury (AKI) with a creatinine level of 218.8umol/L, and the acyclovir and IVIG combination therapy was discontinued. On day 13, the patient was given dexamethasone 15mg/day for five days, followed by oral methylprednisolone (40mg/day). Re-examination of CSF on day 20 showed a decrease in WBC count (10 × 10^6/L vs 73× 10^6/L) and protein (77.46mg/dl vs 175.50mg/dl) levels and an increase in sugar levels (5.04mmol/L vs 4.29mmol/L). The patient was discharged after recovery and had a regular follow-up. Oral methylprednisolone was tapered gradually and then stopped after two months. Three months after symptom onset, the pupils were bilaterally equal and reactive to light, and there was a significant improvement in ocular motility and ptosis. The palpebral fissure was measured at 4 mm on the left (and 8 mm on the right). However, his diplopia persisted. At the 6-month follow-up appointment, his diplopia had completely resolved, and the palpebral fissure was measured at 5mm (Table 1). Patient was satisfied with the treatment.
 |
Table 1 Changes in Clinical Symptoms and Signs Throughout the Treatment Course |
Discussion
Herpes zoster is a common infectious viral disorder with a high incidence rate in the elderly. The eruption caused by herpes zoster usually resolves after 1–2 weeks, while the neurological complications of VZV reactivation can deeply impair patients’ lives. A vaccine has been approved for adult aged 50 and above for the prevention of Herpes Zoster and its neurological complications.5 BBE is a rare disorder of the brainstem and peripheral nervous system and is characterized by an acute (ie, within four weeks) onset of ophthalmoplegia, cerebellar ataxia, and impaired conscious levels, followed by spontaneous recovery about 12 weeks after onset. BEE occurs after an antecedent infection (usually either respiratory and/or gastro-enteric infection). Herpes zoster-associated BBE was first reported in 1993,6 but only two cases have been reported thus far.6,7
There is no gold standard for the diagnosis of BBE. It is mainly diagnosed based on clinical manifestations,8 and other related diseases need to be excluded. In this case, the patient rapidly developed ophthalmoplegia, ataxia, and impairment of consciousness. Other related diseases such as brainstem stroke and tumors were excluded by laboratory and imaging evaluation. The diagnosis of BBE is highly suspected.
Some scholars believe that a positive serum anti-GQ1b antibody is essential for the diagnosis of BBE, but our patient refused to receive the test because of financial limitations. However, a serum IgG of anti-GQ1b antibody cannot be detected in all BBE patients.9 Recent research has shown that the positive rate of serum IgG anti-GQ1b antibody is only 68% in BBE patients.10 This suggests that the BBE diagnosis cannot be excluded even when patients do not have anti-GQib antibodies.
BBE is a demyelinating disease of the central nervous system (CNS) that is caused by a direct viral infection or secondary autoimmune responses. Although a PCR test of CSF VZV-DNA was negative, an intracranial infection cannot be excluded because of his leukocytosis and increased CSF protein levels. In addition, secondary autoimmune responses caused by viral infection may play an important role in the onset of BBE in this patient, and could be responsible for the success of systemic steroid treatment.
Treatment for BBE includes IVIG, systemic steroids, and plasmapheresis, but the value of antiviral treatment is not yet known. In this case, antiviral therapy was continued due to the possibility of intracranial infection and then was stopped after 10 days because of AKI. The serum creatinine level, WBC count and CSF protein level all decreased after acyclovir and IVIG were stopped and systemic steroid therapy was initiated. IVIG could cause AKI because of large amounts of protein passing through the kidney. Sucrose contained in IVIG cannot be metabolized by the kidney, which results in renal tubular necrosis. Thus, laboratory monitoring of renal function is essential. In BBE patients with underlying kidney disease, systemic steroids may be the first-line therapy after intracranial infection is excluded, but clinical studies are required to determine the effectiveness of this treatment.
Conclusions
BBE associated with herpes zoster is very rare and can be overlooked. Dermatologists should be aware of the expanding spectrum of neurological complications caused by VZV infections, which could help to achieve the early diagnosis and treatment.
Ethics Approval and Informed Consent
The authors certify that the patient consent form have been obtained. Written informed consent for publication of the case details including publication of the images was obtained from the patient. The ethics Committee of Beijing Friendship Hospital approved to publish the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Beijing Friendship Hospital, Capital Medical University (contract grant number: seed project YYZZ202128).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Halpern SL, Covner AH. Motor manifestations of herpes zoster; report of a case of associated permanent paralysis of the phrenic nerve. Arch Intern Med. 1949;84(6):907–916. doi:10.1001/archinte.1949.00230060064005
2. Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system - Prognosis, diagnostics and treatment. J Infect. 2015;71(3):281–293. doi:10.1016/j.jinf.2015.06.004
3. Horton E, Krishnamoorthy S, Reynolds L. Bickerstaff’s encephalitis. BMJ Case Rep. 2014;2014(jul30 1):bcr2014205336–bcr2014205336. doi:10.1136/bcr-2014-205336
4. Koga M. ビッカースタッフ型脳幹脳炎の疫学と診断・治療. [Bickerstaff brainstem encephalitis: epidemiology, diagnosis, and therapy]. Nihon Rinsho. 2013;71(5):898–903. Japanese.
5. Al-Khalidi T, Genidy R, Almutawa M, et al. Knowledge, attitudes, and practices of the United Arab Emirates population towards Herpes Zoster vaccination: a cross-sectional study. Hum Vaccin Immunother. 2022;18(5):2073752. doi:10.1080/21645515.2022.2073752
6. Omura A, Watanabe Y, Kobayashi H, et al. A case of Bickerstaff’s encephalitis. With special reference to neurotological findings. Acta Otolaryngol Suppl. 1993;504:125–129. doi:10.3109/00016489309128137
7. Tagawa Y, Yuki N. Bickerstaff’s brainstem encephalitis associated with shingles. J Neurol. 2000;247(3):218–219. doi:10.1007/s004150050567
8. Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. 2001;70(1):50–55. doi:10.1136/jnnp.70.1.50
9. Koga M, Kusunoki S, Kaida K, et al. Nationwide survey of patients in Japan with Bickerstaff brainstem encephalitis: epidemiological and clinical characteristics. J Neurol Neurosurg Psychiatry. 2012;83(12):1210–1215. doi:10.1136/jnnp-2012-303060
10. Ito M, Kuwabara S, Odaka M, et al. Bickerstaff’s brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. 2008;255(5):674–682. doi:10.1007/s00415-008-0775-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.