Back to Journals » OncoTargets and Therapy » Volume 12
A case report for severe hand–foot skin reaction caused by chemotherapy with actinomycin D in a patient with oculocutaneous albinism
Authors Yan SJ, Li Y, Li ZL, Chen Y, Zhang XH, Xiao L
Received 22 November 2018
Accepted for publication 11 February 2019
Published 8 March 2019 Volume 2019:12 Pages 1851—1855
DOI https://doi.org/10.2147/OTT.S195635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shi-Jie Yan,1,2,* Yan Li,3,* Ze-Lian Li,1,2 Ying Chen,1,2 Xiao-Hui Zhang,1,2 Lan Xiao1,2
1Department of Obstetrics and Gynecology, The First Affiliated Hospital, Anhui Medical University, Hefei 230020, Anhui, P.R. China; 2Anhui Province Key Laboratory of Reproductive Health and Genetics, Hefei 230020, Anhui, P.R. China; 3Department of Pathology and Pathophysiology, School of Medicine, Jianghan University, Wuhan 430056, Hubei, P.R. China
*These authors contributed equally to this work
Abstract: Gestational trophoblastic neoplasms (GTN) are highly curable tumors, with an overall patient survival of 90%, due to the individualized chemotherapy. However, chemotherapy regimens vary between different treatment centers and the comparable benefits and risks of these different regimens are unclear. Here, we reported a case of GTN with oculocutaneous albinism (OCA) is resistant to fluorouracil (5-FU), extremely sensitive to actinomycin D (Act-D) with severe hand–foot skin reaction (HFSR). We hypothesized that the known, or unknown, gene mutations might be correlated with drug resistance, supersensitivity and severe drug side effects in OCA patients. Thus, we considered that OCA related genes influence some drug sensitivity and that the absence of melanin likely contributes to some drug resistance. It is important to assess the OCA related gene mutations ocus of drug sensitivity, and resistance in OCA patients in future research.
Keywords: oculocutaneous albinism, gestational trophoblastic neoplasms, actinomycin D, chemotherapy, hand-foot skin reaction
Introduction
Hand–foot skin reaction (HFSR) is the most common and severe adverse reaction after targeted therapy with multitargeted kinase inhibitors (MKIs). It has been reported that the incidence of HFSR with MKIs is as high as 51.4%. Skin lesions of HFSR have shown a series of symptoms that may affect the skin of the hands and/or feet including; burning, erythema, swelling, blister and chapped. According to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0, the severity of HFSR can be divided into the following degrees: degree I, mild skin change or dermatitis (eg, erythema), without pain; degree II, skin change (eg, blister, hemorrhage, edema) or pain, without affecting daily life; degree III, ulcerative dermatitis caused skin burst with pain, seriously impacting daily life.1 Studies have shown that the incidence of HFSR is not related to age, chemotherapy drugs, baseline neuropathy or rash; however, that may be related to the expression of tumor necrosis factor-alpha (TNF-alpha).2
Oculocutaneous albinism (OCA) is an autosomal recessive disorder. The main clinical manifestation is the deficiency of pigment in all parts of the body. The related clinical symptoms cannot be treated so far. It also seriously affects the patients’ physical and mental health. There are no reports concerning potential risks for patients who receive related treatments when they are affected with other diseases.
Act-D is a relatively well-tolerated drug with minimal side effects, even in pediatric patients with Wilms tumor.3 Previous pharmacokinetic studies demonstrated the extensive variation of Act-D exposure in different patients, but the underlying mechanism is unknown.
Case report
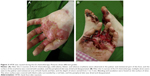
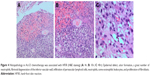
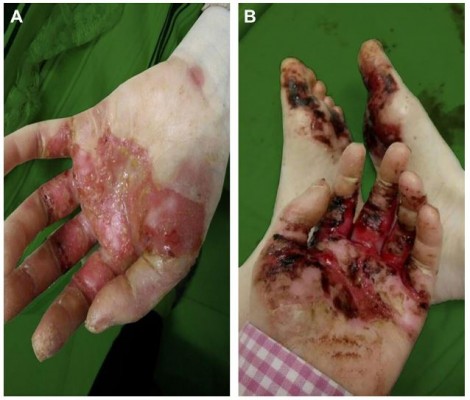
A 26-year-old G2P1 female patient with OCA (Figure 1), due to high β-HCG level (184,122 mIU/mL), uterus curettage was conducted in the local hospital on August 8th, 2017. Pathology demonstrated complete hydatidiform mole based on a limited biopsy. The patient presented at our hospital with a cough and irregular vaginal bleeding lasting for 4 months after debridement of complete hydatidiform mole. The following OCA clinical features were recorded: varying colors of the skin and hair and abnormal ophthalmological findings including; photophobia, nystagmus and reduced visual acuity. The postoperative human chorionic gonadotropin (hCG) level was continuously positive, she was diagnosed with invasive hydatidiform mole by hysteroscopy and curettage pathology combined with multiple metastatic lesions in the lung revealed by CT scan. The patient was classified as a stage III:4 according to FIGO staging and WHO scoring systems. Once the disease was diagnosed, patient chemotherapy with 5-FU (28–30 mg/kg × 10 d) started and was repeated every 2 weeks. This patient was treated with 4 courses of 5-FU chemotherapy. There were no serious adverse events regarding the treatment with 5-FU. The patient did experience grade 1 nausea and vomiting, grade 1 fatigue and mild oral mucositis during the treatment. No HFS or pigmentation developed in this patient. After the 3 courses of 5-FU chemotherapy, the level of β-HCG gradually decreased which indicated potential resistance to 5-FU by the end of the 4 courses. From the 5 courses of chemotherapy, the patient was switched to Act-D chemotherapy (8–10 μg/kg × 8 d), which was also repeated every 2 weeks. After 2 courses of the Act-D chemotherapy, the β-HCG returned to normal levels (Figure 2). On the fifth day of the 2 courses of Act-D chemotherapy, the terminal skin of hand and foot started to show burning sensation and the dry flushing. Three days after finishing of the 2 courses of Act-D chemotherapy, the skin of the hand and foot was slightly swollen, and also blister, erythema, exudate, and pain were observed (Figure 3A). The skin symptoms were improved with urea ointment by local topical application. The patient received the first additional course of Act-D chemotherapy to reduce relapse as scheduled. Only 3 days after finishing of the treatment, the patient suffered from fever and severe HFSR (manifestation of ulcerative dermatitis) (Figure 3B). As shown in the histopathological analysis, a great number of neutrophils, fibrinoid degeneration of the inferior vascular wall, infiltration of perivascular lymphoid cells, neutrophils and some eosinophilic leukocytes, and proliferation of fibroblasts by hematoxylin and eosin (H&E) staining (Figure 4A–C). Accordingly, the patient was diagnosed with degree III HFSR. The above symptoms were rapidly alleviated after appropriate symptomatic treatments.
  | Figure 1 Clinical features of the patient. |
  | Figure 2 Trends in serum β-hCG levels during the patient’s chemotherapy course. |
After treatment completion, the β-HCG titer was tested on a monthly basis. The patient was free of tumor and the β-HCG was negative from early June to November, 2018.
Discussion
HFSR is mainly occurred in cancer patients taking antiangiogenic targeted drugs, while HFS is generally caused by fluorouracil, capecitabine, vinorelbine, and other chemotherapeutic agents.4,5 HFSR and HFS are both characterized by palmoplantar skin reaction. HFSR is characterized by dry skin around the fingers or toes and erythema, whereas HFS is characterized by symmetrical numbness, erythema, and edema. Previous studies have demonstrated that the anti-tumor effect of patients with HFSR after anti-angiogenic therapy was significantly higher than that of the patients without skin reaction.6,7 To date, there has been only one report concerning HFSR in patients with rectal cancer caused by gemcitabine chemotherapy.8
It should be mentioned that the pathogenesis of HFSR is still unclear. The diagnosis of HFSR is mainly based on the medication history, latency, and remission after dosage reduction or withdrawal. So far, no case of HFSR caused by Act-D chemotherapy, as shown in this study, has been reported. The patient in this study had OCA complicated with invasive hydatidiform mole. The related gene mutation caused the melanin deficiency to induce significant changes in the eye and skin. Chemotherapy is required for the treatment of invasive hydatidiform mole. It has been reported that melanin acts as the binding site of some drugs and compounds.9 Related drugs and compounds must be combined with melanin to play an effective role, showing melanin-related side effects, such as pigmentation. Studies have illustrated OCA2 localizes in pigmented structures in mouse melanocytes that bear markers of mature melanosomes, and melanosomes was required for OCA2 function.10,16 Moreover, the study by Cheng et al have shown that UPR attenuation contributes to the extreme chemoresistance phenotype we observed previously in OCA2-null cells.11 In the reports reviewed in this manuscript, no pigmentation and serious adverse events were experienced during the 5-FU course of chemotherapy, and the patient showed 5-FU resistance. After 2 courses of Act-D chemotherapy, the β-hCG level normalized, and the skin reaction appeared between the 2 courses of Act-D chemotherapy. With subsequent treatment courses, the skin reaction gradually worsened. In the last course of Act-D chemotherapy, the skin burst with pain caused by ulcerative dermatitis at the palmar and metatarsal part of the foot was experienced, which seriously affected daily life and was obviously relieved after drug withdrawal. The patient was diagnosed with degree III HFSR. Gene mutations in OCA patients have been previously observed.12,13 In addition, inhibition of melanogenesis can sensitize human melanoma cells toward chemo-, immuno- and radio-therapy.14,15 The presented data may suggest a supersensitivity of OCA patients towards Act-D chemotherapy for OCA mutations. However, due to time constraints in this case, the gene mutations were not evaluated by sequencing. Future studies will further explore whether there is a relationship between OCA gene mutation and HFSR caused by chemotherapy.
Conclusion
In the presented case, according to the patient’s medical history and clinical features, we speculated that OCA patients are relatively insensitive to 5-FU, which may be due to the absence of melanin caused by OCA2 gene mutation and lack of 5-FU binding sites. Loss of OCA2 also disrupts melanogenesis and increases sensitize to Act-D chemotherapy. Thus, OCA patients might get clinical benefits from protective pharmacologic manipulation during Act-D chemotherapy.
Ethical statement
All procedures performed in studies involving the human participant were in accordance with the ethical standards of the First Affiliated Hospital, An Hui Medical University and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Consent for the photos was obtained from the patient. Written informed consent has been provided by the patient to have the case details and any accompanying images published.
Disclosure
The authors report no conflicts of interest in this work.
References
Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. | ||
Lee JH, Chung Y-H, Kim JA, et al. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119(1):136–142. | ||
Gommersall LM, Arya M, Mushtaq I, Duffy P. Current challenges in Wilms’ tumor management. Nat Clin Pract Oncol. 2005;2(6):298–304. | ||
Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13(9):1001–1011. | ||
Cocco M, Pardini S, Podda L, Fozza C, Longinotti M. Prompt recovery of chemotherapy associated hand-foot syndrome treated with acetylsalicylic acid in two patients with Hodgkin’s lymphoma. Eur J Haematol. 2009;82(2):164. | ||
Vincenzi B, Santini D, Russo A, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15(1):85–92. | ||
Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs. 2013;31(4):1078–1086. | ||
Hofheinz RD, Heinemann V, von Weikersthal LF, et al. Capecitabine-associated hand-foot-skin reaction is an independent clinical predictor of improved survival in patients with colorectal cancer. Br J Cancer. 2012;107(10):1678–1683. | ||
Mårs U, Larsson BS. Pheomelanin as a binding site for drugs and chemicals. Pigment Cell Res. 1999;12(4):266–274. | ||
Sitaram A, Piccirillo R, Palmisano I, et al. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol Biol Cell. 2009;20(5):1464–1477. | ||
Cheng T, Orlow SJ, Manga P. Loss of Oca2 disrupts the unfolded protein response and increases resistance to endoplasmic reticulum stress in melanocytes. Pigment Cell Melanoma Res. 2013;26(6):826–834. | ||
Wang Y, Wang Z, Chen M, et al. Mutational analysis of the TYR and OCA2 genes in four Chinese families with oculocutaneous albinism. PLoS One. 2015;10(4):1–10. | ||
Kamaraj B, Purohit R. Mutational analysis on membrane associated transporter protein (MATP) and their structural consequences in oculocutaeous albinism type 4 (OCA4)-A molecular dynamics approach. J Cell Biochem. 2016;117(11):2608–2619. | ||
Brozyna AA, Vanmiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123(6):1448–1456. | ||
Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124(6):1470–1477. | ||
Sitaram A, Dennis MK, Chaudhuri R, et al. Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol Biol Cell. 2012;23(16):3178–3192. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.