Back to Journals » OncoTargets and Therapy » Volume 14
A Case of Phyllodes Tumor of the Breast with Mixed Liposarcoma: Case Report and Literature Review
Authors Tu He Ta Mi Shi ME, Wang N, Yao Q, Dong SS , Feng X, Zhao J, Zou H, Pang LJ, Qi Y
Received 21 December 2020
Accepted for publication 25 February 2021
Published 6 May 2021 Volume 2021:14 Pages 3003—3011
DOI https://doi.org/10.2147/OTT.S298379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Mei Er Tu He Ta Mi Shi,1,* Ning Wang,1,* Qing Yao,1 Shuang-Shuang Dong,1 Xiao Feng,1 Jin Zhao,1 Hong Zou,1 Li-Juan Pang,1 Yan Qi1,2
1Department of Pathology, Shihezi University School of Medicine and the First Affiliated Hospital to Shihezi University School of Medicine, Shihezi, Xinjiang, 832002, People’s Republic of China; 2Department of Pathology, Central People’s Hospital of Zhanjiang and Zhanjiang Central Hospital, Guangdong Medical University, Zhanjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Qi; Li-Juan Pang
Department of Pathology, Shihezi University School of Medicine and The First Affiliated Hospital to Shihezi University School of Medicine, North 2 Road, Shihezi, Xinjiang, 832002, People’s Republic of China
Tel +86 150 09932652
Email [email protected]; [email protected]
Abstract: Phyllodes tumors (PTs) account for less than 1% of breast tumors, and malignant PTs account for even less. Here, we described an unusual case of malignant PT with mixed liposarcoma (myxoid liposarcoma [MLP] and pleomorphic liposarcoma [PLP]). A 52-year-old woman discovered a small lump in her left breast. Twenty years later, the lump suddenly grew within 1 month. Mammography showed space-occupying lesions of the left breast. Histologically, the tumor was characterized by hypercellular stroma covering the epithelium and protrusion of the myoepithelium into the cyst to form a lobulated structure; regions of loose mucus and hypercellular structures alternated. A region of peripheral benign fibroadenoma was also observed, and many stellate and spindle cells or signet ring-like cells were identified in loose areas. Some areas showed a characteristic thin branching vascular pattern. In the cell-rich area, adipocytes and odd megakaryocytes were observed. Atypical mitotic figures were observed in the cell-rich and mucus areas (16 mitoses/10 high-power fields [HPF] and 2 mitoses/10 HPF, respectively). In the immunohistochemical analysis, a small number of tumor cells were positive for AE1/3 and vimentin, whereas all cells were negative for cytokeratin 34βE12, E-cadherin, p63, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and S-100, ruling out the possibility of metaplastic carcinoma. Interestingly, cyclin-dependent kinase 4, mouse double minute 2 (MDM2), and p16 were strongly positive in both loose mucus and cell-rich areas. However, the fluorescence in situ hybridization test results showed that MDM2 was not amplified. Combined with morphological characteristics, these findings supported that the tumor was a mixed malignant PT with MLP and PLP. Our patient did not receive radiation therapy, and after 47 months of follow-up, no recurrence or metastasis occurred. This case report serves to expand the morphologic spectrum of mixed malignant PT with liposarcoma.
Keywords: malignant phyllodes tumor, breast cancer, mixed liposarcoma
Background
Phyllodes tumors (PTs) of the breast are rare fibroepithelial neoplasms accompanied by overgrowth of stromal cells. According to histopathological characteristics, such as tumor margins, mesenchymal cell numbers, interstitial cell atypia, mitotic activity, interstitial overgrowth, and malignant heterogeneous elements, the World Health Organization classifies PTs into benign, borderline, and malignant categories.1 Malignant PTs are rare, accounting for only 0.3–0.5% of malignant breast tumors. Generally, malignant PTs occur in women in their 40s, with most cases occurring between 35 and 55 years of age.2 Malignant PT of the breast (MPTB) can manifest as heterogenic differentiation, stromal cellularity, nuclear pleomorphism, interstitial overgrowth, and more than 10 mitoses figures per 10 high-power fields (HPF). Under these conditions, liposarcomatoid differentiation is most commonly observed. Other types of differentiation include fibrosarcoma, chondrosarcoma, osteosarcoma, and rhabdomyosarcoma.3,4
Herein, we reported the case of a 52-year-old woman with a malignant PT of the breast accompanied by mixed liposarcoma (myxoid liposarcoma [MLP] and pleomorphic liposarcoma [PLP]). We described the clinical, pathological, and immunohistochemical characteristics of this case and performed a literature review. Table 2 lists the reports on PTs and liposarcoma published in recent years.Table 1
 |
Table 1 Antibodies Used of the Immunohistochemical Examination |
 |
Table 2 Review of Reported 19 Cases of Malignant Phyllodes Tumor with Liposarcomatous Differentiation in Breast |
Case Presentation
Clinical History
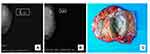
The patient was a 52-year-old woman. She discovered a mass under her left nipple 20 years prior to visiting our hospital. Initially, the mass was the size of an almond, and she did nothing at the time. However, the mass grew suddenly within 1 month prior to her visiting our hospital. Physical examination found a quality of approximately 12 cm × 12 cm in the left breast; the boundary was clear, and the mass was hard. Additionally, no tenderness, superficial varicose veins, nipple depression, nipple discharge after squeezing the breast, and lymph nodes on either side of the axilla were detected. Bilateral mammography examination results showed a mass in the left outer upper quadrant, which showed increased shadow density and multiple calcification shadows. Imaging of space-occupying lesions in the left breast was performed using the Breast Imaging-Reporting and Data System (BI-RADS) classification into three categories (Figure 1A). After observation in the hospital, radical mastectomy of the unilateral breast (left breast) was performed. Intra-operative freezing reports revealed mammary stromal PTs with obvious cell atypia, and a diagnosis of PT was considered.
The excised specimens were fixed with 10% neutral formalin and processed routinely. Paraffin-embedded blocks were sectioned into 5 μm-thick sections, which were then stained with hematoxylin and eosin (H&E). Paraffin-embedded tissue samples were also used for immunohistochemical analysis. Table 1 lists the antibodies, clones, and working dilutions in this study, along with the commercial sources of these reagents. The surgical specimen was breast tissue with spindle skin and nipple, measuring 15 cm × 11 cm × 6.5 cm. On the cut surface, a nodule measuring 8 cm × 6 cm × 5.5 cm was found under the nipple and showed clear boundaries with surrounding tissue (Figure 1C). The nodule was solid and cystic, and the cut surface was gray and grayish yellow, showing a fleshy appearance lobulated with fissures similar to leaf buds. Some areas displayed cystic changes.
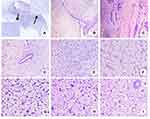
Histologically, the tumor showed an invasive boundary. The tumor had loose and dense cell areas (Figure 2A), a cell-rich mesenchyme covered the epithelium, and the myoepithelium protruded into the sac cavity to form a leaf-like structure under low magnification (Figure 2B). Residual normal ducts were rarely observed, and loose mucus areas alternated with cell-rich areas. Fibroadenoma areas could be seen around the cell-rich area (Figure 2C). Residual ducts were surrounded by a cell-rich area (Figure 2D). In the loose mucus area, there were many stellate cells and spindle cells (Figure 2E), and there was a characteristic thin, branching vascular pattern, resembling “chicken wire.” The cells around the blood vessels were mostly round, oval, or short spindle-shaped primitive mesenchymal cells, essentially demonstrating the same shape (Figure 2F). There were more adipocytes and oddly shaped megakaryocytes in the cell-rich area (Figure 2G), and different degrees of interstitial collagenization were detected. Moreover, the cells showed obvious atypia and were arranged into rosettes (Figure 2I). There were more nuclear divisions, particularly for megakaryocytes in high-density areas, with a mitotic index of 16 mitoses/10 HPF. In the mucus areas, mild nuclear atypia was observed, and the mitotic index was 2 mitoses/10 HPF. The nuclei were short, spindle-shaped, and vacuolated.
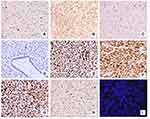
Based on immunostaining, only a few tumor cells were positive for AE1/3 (Figure 3A) and vimentin (Figure 3B), whereas staining for cytokeratin (CK) 34βE12, E-cadherin (Figure 3C), p63, and S-100 (Figure 3D) was negative, supporting the diagnosis of PT. The lack of CK34βE12 and p63 staining confirmed that the tumor was not metaplasia. Tumor mesenchymal cells were positive for vimentin (Figure 3B) and smooth muscle actin, suggesting that tumor cells were the source of mesenchymal cells. Interestingly, immunohistochemical staining was diffusely positive for p16 (Figure 3E), mouse double minute 2 (MDM2; Figure 3F), and cyclin-dependent kinase 4 (CDK4; Figure 3G). Additionally, proliferating cells were positive for the proliferative marker Ki-67, and the labeling index was more than 90% (Figure 3H). However, the fluorescence in situ hybridization (FISH) test results showed that MDM2 was not amplified (Figure 3I). Thus, based on histological analysis of various markers combined with morphological analysis, we obtained a pathological diagnosis of malignant PT. We performed a modified radical mastectomy on the left breast, and the patient recovered well after the operation.
Discussion
Patients with PT typically complain of breast lumps and harbor rapidly growing painless masses.5 The most common location of involvement is the upper lateral quadrant. However, several reports of multifocal and bilateral tumors have also been described in the literature.6,7 It is not easy to draw a clear line between PT and fibroadenoma, and conducting a rigorous pathological assessment, including a combination of histologic features, immunohistochemical staining, and exact clinical information, is necessary to obtain an accurate diagnosis. Other studies have shown that these tumors have significant heterogeneity, and CDKN2A, HOXB13, PAX3, SIX1, HMGA3, TGFB2, and other genes may play important roles in the malignant progression of PT.8 Recent data suggest that malignant PT cells exhibit mesenchymal stem cell characteristics and can be induced or spontaneously differentiated into other lineages, consistent with previous reports on the heterogenic differentiation of PT.9 Moreover, malignant PT is common with well-differentiated liposarcoma (WDL) or dedifferentiated liposarcoma (DDL), whereas malignant PT with MLP and PLP is rare. To date, only three cases of PT with mixed differentiation of sarcoma have been reported; one was malignant PT mixed with differentiation of MLP and PLP;10 another was malignant PT mixed with differentiation of osteosarcoma, chondrosarcoma, and liposarcoma;11 and the remaining one was malignant PT mixed with WLD and MLP.12 In our case, malignant PT was accompanied by MLP and PLP.
Imaging findings cannot distinguish fibroadenomas from benign, borderline, and malignant PTs. In the differential diagnosis of benign, borderline, or malignant PTs, B-ultrasound and molybdenum targets have shown a large number of overlapping image features of benign, borderline, or malignant PTs.13 According to previous studies, PTs are usually irregularly shaped and larger than fibroadenomas, which are typically oval-shaped on mammograms and ultrasounds. Additionally, fibroadenomas have been reported in patients younger than 30 years of age,14 whereas PTs are more common in older patients (eg, 35–55 years of age).15 Malignant PT is characterized by marked stromal cellularity and nuclear pleomorphism, stromal overgrowth, and more than 10 mitoses/10 HPF. The presence of heterologous sarcomas (eg, liposarcoma, chondrosarcoma, and osteosarcoma) can be identified as malignant PT. The abundance of blood vessels may be an important feature of PT, whereas fibroadenomas typically have fewer blood vessels. Studies have also shown that fibroadenomas are homogeneous and hypoechoic, whereas PTs are complex and heterogeneous. Notably, 50–77% of PTs exhibit the posterior sound enhancement phenomenon as a feature of ultrasound. Additionally, the high density of lesions could be a useful mammographic feature suggestive of PTs owing to the larger lesion size. In recent studies, enhanced magnetic resonance imaging of malignant lobulated tumors showed angiogenesis and internal cystic space.16 However, these imaging methods do not yield accurate diagnoses, and the final diagnosis depends on surgical resection and pathological examination. In general, PTs appear as clear, firm, multinodular masses. The cutting surface is off-white, and the appearance is uniform. Visible mucus-like areas, cystic cavities, bleeding, and necrotic areas are also detected.17 In a study by Kim et al18 the average tumor diameter was found to be 4 cm; those of benign, borderline, and malignant PTs were 3.7, 4.2, and 6.2 cm, respectively. Another study19 showed that the average diameters of benign, borderline, and malignant PTs were 3.2, 5.06, and 4.6 cm, respectively. According to a literature review, the general diameter of malignant lobar tumors is greater than 3 cm. In our case, the tumor diameter was 8 cm, which was quite large.
A few reports have described the differentiation of malignant PT combined with liposarcoma. The main treatment for malignant PT is extensive local resection or total mastectomy. In recent years, cases of breast conservative surgery have increased.5,19,20 Surgical margins have been found to be important for predicting recurrence in some studies, and most authors have chosen 10 mm as the optimal margin.19,21–23 However, this wide margin results in poor cosmetic results, and total mastectomy remains a good choice.3 Owing to the low rate of lymph node involvement, no axillary lymph node dissection is required.24–26 The study conducted by Gnerlich et al27 was the largest analysis investigating the role of adjuvant radiotherapy (RT) in MPTB patients. It included 3120 patients with MPTB, of which 14.3% received adjuvant RT. They found that adjuvant RT significantly reduced local recurrence (adjusted hazard ratio: 0.43, 95% confidence interval: 0.19–0.95). In other retrospective studies, the local control rate of patients receiving adjuvant RT was higher when compared to those only undergoing surgery, but the characteristics between groups were significantly uneven.28,29 Many authors have noted that in MPTB treated with BCS (breast conserving surgery) or total mastectomy with tumor-free margins < 1 cm, adjuvant irradiation is indicated.30–33 The largest analysis conducted by Gnerlich et al27 showed that adjuvant RT had no effect on disease-free survival or overall survival. In our case, after 47 months of follow-up, the patient did not receive any other relevant treatment, and there was no recurrence or metastasis.
The difference between malignant PT and metaplastic carcinoma lies in morphology. Metaplastic carcinoma may also appear as spindle cells with nuclear pleomorphism, abundant mitotic figures, and heterologous components. The presence of leaf-like structures and a mild epithelial cell lining are typical of PT. The malignant epithelial component, if present, tends to be a metaplastic carcinoma. If there is no epithelial component, particularly in the core biopsy, immunohistochemistry may be useful. CKs (eg, CKAE1/AE3, CK5/6, 34bE12, and Cam5.2) and the myoepithelial marker p63 should be used for the examination because the staining pattern of metaplastic carcinoma is different from that of PT, and most CKs and p63 will be negative in PTs.34
Malignant PTs may be confused with primary liposarcoma of the breast. Both are diseases of interstitial cell proliferation. The diagnosis of these lesions depends on the detection of residual epithelial structures, which are present in PTs but not in primary breast tumors and rarely in primary sarcomas of the breast. Although the clinical features of both lesions are similar, PT with liposarcoma differentiation is associated with a better prognosis than primary liposarcoma of the breast.35 In our case, no further treatment was necessary, and no recurrence or metastasis was found after 47 months of follow-up. Therefore, we believe that our case was likely to be malignant PT with liposarcoma differentiation.
P16 and p14ARF (p14) are tumor-suppressor proteins encoded by CDKN2A. P14 binds to MDM2 and inhibits its ubiquitin ligase activity, thereby increasing the expression of the anti-apoptotic protein p53. P16 binds to the CDK4/cyclin D1 complex to inhibit cell cycle progression. Overexpression of MDM2 and CDK4 is thought to trigger CDKN2A feedback, thereby inducing the transcription of p14 and p16 proteins and blocking cell proliferation. Accordingly, p16 expression levels are thought to be correlated with MDM2 and CDK4 levels and may be a promising marker for the diagnosis of liposarcoma.36 MDM2 and CDK4 proteins are strongly positive and/or show gene amplification in 90% of primary WDLs/DDLs,9 and 83% of ALT/WDLs showed positive reactivity with p16.37 However, Lyle et al4 found that PTs with liposarcoma differentiation do not express MDM2 or CDK4 and show no gene amplification in WDLs/DDLs. Interestingly, immunohistochemical staining for MDM2, CDK4, and p16 in the MLP and PLP areas in our case was diffusely positive, potentially because of the pathogenesis of malignant PT with mixed liposarcoma differentiation, but the FISH test results showed that MDM2 was not amplified. An MDM2/CEP12 ratio > 2 is considered amplified for the MDM2 gene;38 therefore, the results were more consistent with our diagnosis of PT with MLP and PLP differentiation. Liu et al39 identified mutually exclusive activation hotspot mutations in the phosphatidylinositol 3-kinase/RAS signal transduction gene associated with fibroblast growth factor receptor 1 in malignant PTs and identified other driving factors, such as TERT promoter, TP53, MED12, and the protein methyltransferases SETD2 and KMT2D, that are related to the pathogenesis and/or progression of tumors. No specific genomic characteristics of liposarcoma differentiation have been found; however, the heterologous and non-heterologous components of malignant PTs showed different signs of evolution, which may have clinical significance for clonal selection and tumor progression. Recently, a novel genome has been used as an auxiliary diagnostic tool to characterize and analyze breast fibroepithelial lesions,14 indicating that this gene detection and prediction model may be used as an auxiliary tool to diagnose breast epithelial lesions in the clinical setting using core biopsy tissues.
In conclusion, our results highlighted the morphological characteristics of PTs with MLP and PLP, and we reviewed the literature describing the differentiation of PTs with liposarcoma, which cannot be confirmed by imaging alone. Our findings showed that diagnosis of this tumor should be performed using a combination of clinical, pathological, and immunohistochemical staining results. The main treatment for malignant PT is extensive local resection or total mastectomy.
Consent to Publish
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. The images do not contain patient records or information. This study was approved by the Clinical Research Ethics board of the First Affiliated Hospital, Shihezi University School of Medicine (Shihezi, China).
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81860471), the International Cooperation Projects of Shihezi University (grant no. GJHZ201710), and the Postgraduate Case Library Construction Project of Shihezi University (grant no. 2019Y-AL05).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. World Health Organization (WHO) Classification of tumours of the breast. In: WHO Classification of Tumours. Vol. 4,
2. Nistor-Ciurba CC, Buiga R, Popiţa C, Eniu DT, Puşcaş ME, Ignat FL. A malignant phyllodes tumor with liposarcomatous differentiation case with 3-year follow-up. Romanian J Morphol Embryol/Revue Roumaine De Morphologie Et Embryologie. 2019;60(3):979–983.
3. Tan BY, Acs G, Apple SK, et al. Phyllodes tumours of the breast: a consensus review. Histopathology. 2016;68(1):5–21. doi:10.1111/his.12876
4. Lyle PL, Bridge JA, Simpson JF, Cates JM, Sanders ME. Liposarcomatous differentiation in malignant phyllodes tumours is unassociated with MDM2 or CDK4 amplification. Histopathology. 2016;68(7):1040–1045. doi:10.1111/his.12898
5. Zhao H, Cheng X, Sun S, Yang W, Kong F, Zeng F. Synchronous bilateral primary breast malignant phyllodes tumor and carcinoma of the breast with Paget’s disease: a case report and review of the literature. Int J Clin Exp Med. 2015;8(10):17839–17841.
6. Karczmarek-Borowska B, Bukala A, Syrek-Kaplita K, Ksiazek M, Filipowska J, Gradalska-Lampart M. A rare case of breast malignant phyllodes tumor with metastases to the kidney: case report. Medicine. 2015;94(33):e1312. doi:10.1097/MD.0000000000001312
7. Mallory MA, Chikarmane SA, Raza S, Lester S, Caterson SA, Golshan M. Bilateral synchronous benign phyllodes tumors. Am Surg. 2015;81(5):E192–194. doi:10.1177/000313481508100503
8. Ang MK, Ooi AS, Thike AA, et al. Molecular classification of breast phyllodes tumors: validation of the histologic grading scheme and insights into malignant progression. Breast Cancer Res Treat. 2011;129(2):319–329. doi:10.1007/s10549-010-1204-5
9. Inyang A, Thomas DG, Jorns J. Heterologous liposarcomatous differentiation in malignant phyllodes tumor is histologically similar but immunohistochemically and molecularly distinct from well-differentiated liposarcoma of soft Tissue. Breast J. 2016;22(3):282–286. doi:10.1111/tbj.12567
10. Kim JM, Jung EJ, Kim JY, et al. A rare case of mixed type liposarcoma of breast arising in malignant phyllodes tumor. Breast J. 2020;26(2):271–273. doi:10.1111/tbj.13546
11. Tomas D, Bujas T, Stajduhar E, Kirac P, Mijić A, Kruslin B. Malignant phyllodes tumor with associated osteosarcomatous, chondrosarcomatous, and liposarcomatous overgrowth. APMIS. 2007;115(4):367–370. doi:10.1111/j.1600-0463.2007.apm_588.x
12. Lee WY, Cheng L, Chang TW. Fine needle aspiration cytology of malignant phyllodes tumor with liposarcomatous stroma of the breast. A case report. Acta Cytol. 1998;42(2):391–395. doi:10.1159/000331624
13. Kalambo M, Adrada BE, Adeyefa MM, et al. Phyllodes tumor of the breast: ultrasound-pathology correlation. AJR Am J Roentgenol. 2018;210(4):W173–W179. doi:10.2214/AJR.17.18554
14. Sim Y, Ng GXP, Ng CCY, et al. A novel genomic panel as an adjunctive diagnostic tool for the characterization and profiling of breast Fibroepithelial lesions. BMC Med Genomics. 2019;12(1):142. doi:10.1186/s12920-019-0588-2
15. Rodrigues MF, Truong PT, McKevitt EC, Weir LM, Knowling MA, Wai ES. Phyllodes tumors of the breast: the British Columbia Cancer Agency experience. Cancer Radiotherapie. 2018;22(2):112–119. doi:10.1016/j.canrad.2017.08.112
16. Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care (Basel, Switzerland). 2016;11(2):123–127. doi:10.1159/000444377
17. Venter AC, Roşca E, Daina LG, Muţiu G, Pirte AN, Rahotă D. Phyllodes tumor: diagnostic imaging and histopathology findings. Romanian J Morphol Embryol/Revue Roumaine De Morphologie Et Embryologie. 2015;56(4):1397–1402.
18. Kim S, Kim JY, Kim DH, Jung WH, Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 2013;141(3):353–363. doi:10.1007/s10549-013-2684-x
19. Hasdemir S, Tolunay Ş, Özşen M, Gökgöz MŞ. Phyllodes tumor of the breast: a clinicopathological evaluation of 55 cases. Eur j Breast Health. 2020;16(1):32–38. doi:10.5152/ejbh.2019.4709
20. Mituś J, Reinfuss M, Mituś JW, et al. Malignant phyllodes tumor of the breast: treatment and prognosis. Breast J. 2014;20(6):639–644. doi:10.1111/tbj.12333
21. Onkendi EO, Jimenez RE, Spears GM, Harmsen WS, Ballman KV, Hieken TJ. Surgical treatment of borderline and malignant phyllodes tumors: the effect of the extent of resection and tumor characteristics on patient outcome. Ann Surg Oncol. 2014;21(10):3304–3309. doi:10.1245/s10434-014-3909-x
22. Lin CC, Chang HW, Lin CY, Chiu CF, Yeh SP. The clinical features and prognosis of phyllodes tumors: a single institution experience in Taiwan. Int J Clin Oncol. 2013;18(4):614–620. doi:10.1007/s10147-012-0442-4
23. Mangi AA, Smith BL, Gadd MA, Tanabe KK, Ott MJ, Souba WW. Surgical management of phyllodes tumors. Arch Surg. 1999;134(5):
24. Padmanabhan V, Dahlstrom JE, Chong GC, Bennett G. Phyllodes tumor with lobular carcinoma in situ and liposarcomatous stroma. Pathology. 1997;29(2):224–226. doi:10.1080/00313029700169924
25. Guillot E, Couturaud B, Reyal F, et al. Management of phyllodes breast tumors. Breast J. 2011;17(2):129–137. doi:10.1111/j.1524-4741.2010.01045.x
26. Gullett NP, Rizzo M, Johnstone PA. National surgical patterns of care for primary surgery and axillary staging of phyllodes tumors. Breast J. 2009;15(1):41–44. doi:10.1111/j.1524-4741.2008.00669.x
27. Gnerlich JL, Williams RT, Yao K, Jaskowiak N, Kulkarni SA. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Data Base, 1998-2009. Ann Surg Oncol. 2014;21(4):1222–1230. doi:10.1245/s10434-013-3395-6
28. Xu B, Hu X, Jiang Z, et al. National consensus in China on diagnosis and treatment of patients with advanced breast cancer. Ann Transl Med. 2015;3(17):242. doi:10.3978/j.issn.2305-5839.2015.09.47
29. McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (London, England). 2014;383(9935):2127–2135. doi:10.1016/S0140-6736(14)60488-8
30. Barth RJ, Wells WA, Mitchell SE, Cole BF. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009;16(8):2288–2294. doi:10.1245/s10434-009-0489-2
31. Pezner RD, Schultheiss TE, Paz IB. Malignant phyllodes tumor of the breast: local control rates with surgery alone. Int J Rad Oncol Biol Phys. 2008;71(3):710–713. doi:10.1016/j.ijrobp.2007.10.051
32. Soumarová R, Seneklová Z, Horová H, et al. Retrospective analysis of 25 women with malignant cystosarcoma phyllodes–treatment results. Arch Gynecol Obstet. 2004;269(4):278–281. doi:10.1007/s00404-003-0593-7
33. Theriault RL, Carlson RW, Allred C, et al. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Comprehensive Cancer Net. 2013;11(7):
34. Zhang Y, Kleer CG. Phyllodes tumor of the breast: histopathologic features, differential diagnosis, and molecular/genetic updates. Arch Pathol Lab Med. 2016;140(7):665–671. doi:10.5858/arpa.2016-0042-RA
35. Moffat CJ, Pinder SE, Dixon AR, Elston CW, Blamey RW, Ellis IO. Phyllodes tumours of the breast: a clinicopathological review of thirty-two cases. Histopathology. 1995;27(3):205–218. doi:10.1111/j.1365-2559.1995.tb00212.x
36. Kammerer-Jacquet SF, Thierry S, Cabillic F, et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum Pathol. 2017;59:34–40. doi:10.1016/j.humpath.2016.08.009
37. He M, Aisner S, Benevenia J, Patterson F, Aviv H, Hameed M. p16 immunohistochemistry as an alternative marker to distinguish atypical lipomatous tumor from deep-seated lipoma. Appl Immunohistochem Mol Morphol. 2009;17(1):51–56. doi:10.1097/PAI.0b013e3181719223
38. Weaver J, Downs-Kelly E, Goldblum JR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Modern Pathol. 2008;21(8):943–949. doi:10.1038/modpathol.2008.84
39. Liu SY, Joseph NM, Ravindranathan A, et al. Genomic profiling of malignant phyllodes tumors reveals aberrations in FGFR1 and PI-3 kinase/RAS signaling pathways and provides insights into intratumoral heterogeneity. Modern Pathol. 2016;29(9):1012–1027. doi:10.1038/modpathol.2016.97
40. Narla SL, Stephen P, Kurian A, Annapurneswari S. Well-differentiated liposarcoma of the breast arising in a background of malignant phyllodes tumor in a pregnant woman: A rare case report and review of literature[J]. Indian journal of pathology & microbiology. 2018;61(4):577–579
41. Hallin M, Thway K. Phyllodes Tumor With Heterologous Liposarcomatous Differentiation[J]. International journal of surgical pathology. 2017;25(5):435–437
42. Uriev L, Maslovsky I, Vainshtein P, Yoffe B, Ben-Dor D. Malignant phyllodes tumor with heterologous liposarcomatous differentiation and tubular adenoma-like epithelial component[J]. International journal of medical sciences. 2006;3(4):130–134
43. Isotalo PA, George RL, Walker R, Sengupta SK. Malignant phyllodes tumor with liposarcomatous differentiation[J]. Archives of pathology & laboratory medicine. 2005;129(3):421-42
44. Kuroda N, Sugimoto T, Ueda S, Takahashi T, Moriki T, Sonobe H, et al. Malignant phyllodes tumor of the breast with expression of osteonectin and vinculin[J]. Pathology international. 2001;51(4):277-282
45. Satou T, Matsunami N, Fujiki C, Tanaka K, Hayashi Y, Hashimoto S. Malignant phyllodes tumor with liposarcomatous components: a case report with cytological presentation[J]. Diagnostic cytopathology. 2015;11(4):1032
46. Sancheti SM, Sawaimoon SK, Ahmed R. Pleomorphic liposarcoma arising in a malignant phyllodes tumor of breast: A rare occurrence[J]. Journal of cancer research and therapeutics. 2015;11(4):1032
47. Lee JW, Nadelman CM, Hirschowitz SL, Debruhl ND, Bassett LW. Malignant phyllodes tumor of a genotypic male, phenotypic female with liposarcomatous differentiation[J]. The breast journal. 2007;13(3):312-313
48. Vera-Alvarez J, Marigil-Gómez M, Abascal-Agorreta M, García-Prats MD, López-López JI, Pérez-Ruiz J. Malignant phyllodes tumor with pleomorphic liposarcomatous stroma diagnosed by fine needle aspiration cytology: a case report[J]. Acta cytologica. 2002;46(1):50-56
49. Scala M, Mereu P, Comandini D, Nocentini L, Vecchio C. [Malignant phyllodes tumor with liposarcomatous differentiation. Description of a clinical case][J]. Minerva chirurgica. 1994;54(5):355-358
50. Isimbaldi G, Sironi M, Declich P, Galli C, Assi A. A case of malignant phyllodes tumor with muscular and fatty differentiations[J]. Tumori Tumori. 1992;78(5):351-352
51. De Luca LA, Traiman P, Bacchi CE. An unusual case of malignant cystosarcoma phyllodes of the breast[J]. Gynecologic oncology. 1986;24(1):91-96
52. Jimenez JF, Gloster ES, Perrot LJ, Mollitt DL, Gollady ES. Liposarcoma arising within a cystosarcoma phyllodes[J]. Journal of surgical oncology. 1986;31(4):294-298
53. Qizilbash AH. Cystosarcoma phyllodes with liposarcomatous stroma[J]. American journal of clinical pathology. 1976;65(3):321-327
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.