Back to Journals » Infection and Drug Resistance » Volume 12
A case of persistent bacteraemia by Ralstonia mannitolilytica and Ralstonia pickettii in an intensive care unit
Authors Basso M, Venditti C, Raponi G , Navazio AS, Alessandri F, Giombini E, Nisii C , Di Caro A , Venditti M
Received 22 February 2019
Accepted for publication 12 June 2019
Published 2 August 2019 Volume 2019:12 Pages 2391—2395
DOI https://doi.org/10.2147/IDR.S206492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sahil Khanna
Monica Basso,1,* Carolina Venditti,2,* Giammarco Raponi,3 Anna Sara Navazio,3 Francesco Alessandri,4 Emanuela Giombini,2 Carla Nisii,2 Antonino Di Caro,2 Mario Venditti3
1Department of Molecular Medicine, University of Padova, Padova, Italy; 2Laboratory of Microbiology, L. Spallanzani National Institute for Infectious Diseases, Rome, Italy; 3Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome Italy; 4Department of Anesthesia and Intensive Care Medicine, Sapienza University of Rome, Rome, Italy
*These authors contributed equally to this work
Abstract: The Ralstonia spp. genus is a group of non-fermentative, Gram-negative bacteria often resistant to many antibiotics, which are emerging as opportunistic pathogens frequently associated with infections in hospital settings. We present herein a case of combined R. pickettii and R. mannitolilytica persisting and relapsing bacteraemia, possibly caused by a septic arterial thrombosis secondary to the rupture of an internal carotid artery aneurysm. Microbiology studies showed that both Ralstonia isolates produced biofilm and carried class D oxacillinase genes. When confronted with infections caused by members of the Ralstonia genus, identification to the species level is crucial for correct clinical management, as the two species show different antibiotic susceptibility patterns.
Keywords: Ralstonia pickettii, Ralstonia mannitolilytica, bacteraemia, endovascular infection
Background
Members of the Ralstonia genus are emerging opportunistic pathogens that include R. pickettii, R. mannitolilytica, R. solanacearum, and R. insidiosa. R. pickettii and R. mannitolilytica have been involved in a wide spectrum of infections in hospital settings.1–4 Frequent reports of infections associated with these non-fermenting Gram-negative rods, as well as their resistance to many classes of antibiotics, have determined a rise in attention for these pathogens in recent years.5–7
We describe herein a very unusual case of persisting and relapsing bacteraemia caused by both R. pickettii and R. mannitolilytica, probably secondary to an endovascular infection.
Case report
A 46-year-old female with a medical history of breast implants and thyroidectomy was admitted on 25 March 2018 to the Emergency Department of the Policlinico Umberto I in Rome, Italy, for headache, vomiting and neurological deterioration with hyposthenia of the left hemysome. On admission, a cerebral CT scan showed an intraparenchymal hemorrhage of the right frontoparietal region. Magnetic resonance imaging (MRI) of the brain (with gadolinium) evidenced the rupture of an internal carotid artery aneurysm. After emergency evacuation of the haematoma and clipping of the aneurysm, the patient was transferred to the intensive care unit (ICU). On day 6, the patient developed fever up to 38.5 °C, and was treated for 5 days with intravenous gentamicin for an Escherichia coli urinary tract infection. On day 11, fever up to 39 °C reappeared without hemodynamic deterioration and no worsening of respiratory exchanges. Blood examinations showed neutrophilic leukocytosis, thrombocytopenia, and a procalcitonin value of 1.15 ng/mL (normal value <0.5 ng/mL). Multiple blood cultures revealed the growth of R. mannitolilytica and R. pickettii. Laboratory results showed that both isolates were susceptible to ciprofloxacin and trimethoprim-sulfamethoxazole, however fever persisted despite targeted therapy with intravenous ciprofloxacin (400 mg tid). On day 19, additional blood cultures were performed, which became positive for Candida parapsilosis, R. pickettii and R. mannitolilytica. Cultures of the removed central venous catheter, respiratory secretions, urine and rectal swab were all negative for Ralstonia spp. A transthoracic echocardiogram showed no signs of cardiac vegetations and funduscopic examinations were also negative. Conversely, MRI showed thrombosis of the internal carotid artery upstream of the surgical clip, which required anticoagulant therapy with enoxaparin 6000 IU bid for three months. Clearance of candidemia was obtained after 4 days of antifungal therapy; trimethoprim-sulfamethoxazole (5 mg/kg of trimethoprim iv q6 h) was added to the antimicrobial regimen on day 25 because blood cultures were still positive for both Ralstonia species. The antibiotic treatment was discontinued on day 40. However, R. pickettii and R. mannitolilytica bacteraemia relapsed a week later and the patient was again treated with a six-week course of trimethoprim-sulfametoxazole and ciprofloxacin. On day 52, the patient underwent cranioplasty surgery without complications. On day 80, the patient developed fever, and R. pickettii was once again grown from blood cultures. Ciprofloxacin (400 mg iv tid) was administered; a progressive clinical improvement was observed and the patient was transferred to a rehabilitation centre where she completed an eight-week therapy course. No further relapse of Ralstonia infection was observed after a six-month follow up.
Culture and characterization of strains
Blood cultures were incubated in a BACT/ALERT® VIRTUO® system (bioMérieux, Marcy-l’Étoile, France) at the Clinical Microbiology laboratory of the Policlinico Umberto I in Rome, Italy. Positive blood cultures were seeded on solid media for 48 hrs, and colony identification was performed using the Vitek-2 automated system (bioMérieux), which identified the Ralstonia genus. The MALDI TOF MS (bioMérieux) technique on the other hand yielded a rapid and accurate species-level identification of two distinct species: R. mannitolilytica and R. pickettii (confidence values of 99%). These identification results were subsequently confirmed by 16S rDNA gene sequencing.
Antimicrobial susceptibility was performed by the broth microdilution method (MicroScan WalkAway plus, Beckman Coulter, Milan, Italy). As there are no EUCAST susceptibility breakpoints available for Ralstonia spp., MIC results were interpreted using the criteria used for Pseudomonas spp and Acinetobacter spp. for trimethoprim-sulfamethoxazole.8 As shown in Table 1, the two Ralstonia species showed different resistance profiles: R. mannitolilytica was resistant to β-lactams and aminoglycosides, but susceptible to piperacillin/tazobactam, while R. pickettii was resistant to piperacillin/tazobactam and most of the β-lactams, but susceptible to imipenem and aminoglycosides. Both strains were negative for the presence of carbapenemase genes (blaKPC, blaNDM, blaOXA-48, and blaVIM), as determined by RT-PCR (GeneXpert, Cepheid, USA).
 |
Table 1 MIC values for the Ralstonia spp. clinical isolates described in this study |
Antimicrobial resistance genes were also investigated by Whole Genome Sequencing (WGS) using the Ion Torrent GSS5 platform (Life Technologies, Carlsbad, California, USA) by constructing single-end libraries with an average length of 200bp, according to manufacturer’s instructions. All raw reads generated were submitted to the Sequence Read Archive (SRA) under the BioProject accession number SRP154097.
The resistance genes were extracted from the WGS data using the ResFinder v3.0 webserver (http://www.genomicepidemiology.org). The minimum percentage of sequence identity was set at 100%, with an alignment length of >98%. Both strains carried the resident narrow-spectrum oxacillinase blaOXA-22; R. pickettii also harboured blaOXA-60, an inducible oxacillinase with carbapenem-hydrolyzing property. In addition to the blaOXA-22 gene, R. mannitolilytica showed the presence of the intrinsically species-specific class D oxacillinase blaOXA-443 and blaOXA-444 genes, and the serin-hydrolase class C family beta-lactamase (GenBank Accession number WP_045219476). Biofilm production capacity was evaluated by BioTimer-resazurin assay (Canvax Biotech, Spain) as previously described.9 Both isolates produced biofilm in a dose-dependent manner with bacterial concentrations as low as 75 live cells/mL.
Discussion
Emerging opportunistic pathogens such R. pickettii and R. mannitolilytica have been shown to cause several infections, especially in immunosuppressed patients. R. pickettii was recognized as a causative agent of infection of the seminal tract, of bones and joints (including prosthetic joints), as well as a cause of infective endocarditis, severe pneumonia and fulminant sepsis.10–14 R. mannitolilytica on the other hand has been described as a cause of recurrent ventricular-atrial shunt-associated meningitis.4 The ability of these bacteria to cause localized and systemic infections is likely due to their ability to produce biofilm, which in turn is key to their survival in the environment, their evasion of the host’s immune response and their frequent antibiotic resistance.15,16 The clinical presentation of both species is also similar, and both have been described as agents of bacteraemia, especially in high-risk patients.7,17,18
To the best of our knowledge, this is the first report of persisting and relapsing bacteraemia caused by two distinct Rastonia species in an immunocompetent patient. Our case concerns a patient without any apparent comorbidity or predisposing condition who developed the first episode of bacteraemia by the two Ralstonia species six days after the clipping of an internal carotid aneurysm. One possibility is that the infection was acquired following contamination during or after the neurosurgical clipping procedure. Indeed, Ralstonia bacteria have been associated with infections in hospital settings due to their ability to survive in liquid media (including saline and chlorhexidine with 0.05% aqueous solutions) and on hospital devices.19 In our case, environmental samples of air or from inanimate surfaces taken monthly in the operating rooms and in all areas where the patient had been treated were negative for Ralstonia spp; this is not surprising as even in cases of well documented hospital outbreaks the environmental origin was not identified.20,21 The case we have described was also the only occurrence in the hospital, supporting the hypothesis that no environmental contamination had taken place. Furthermore, an infection related to the use of an intravascular device was also ruled out, since cultures of the removed central venous catheter did not show any growth of Ralstonia species. An alternative hypothesis was based on the study by Ziganshina et al,22 who demonstrated that members of the genera Ralstonia are one of the most significant taxa identified in atherosclerotic plaques removed during surgery. Thus, we could speculate that either the aneurysm rupture or the subsequent neurosurgical management might have provided access to an anatomically protected site. Unfortunately, atheroma material was not available to confirm this hypothesis. Regardless of the source of the infection, a combination of arterial thrombosis and biofilm-related chronic contamination, could have represented a key factor for the persistence and relapse of the Ralstonia spp. bacteraemia, which occurred despite an appropriate and long-term antibiotic therapy.23,24
The antimicrobial treatment and management of Ralstonia spp. infections is challenging, firstly because of the difficulty in correctly identifying and differentiating between Ralstonia spp. members using routine biochemical methods.7,17 Although the 16S rDNA is described as the reference method, in our case the mass spectrometry yielded a rapid and accurate species-level identification, in accordance with other studies.21,25 Ralstonia species are also resistant to different antimicrobial agents, mainly β-lactams (including carbapenems) given the presence of the class D β-lactamases blaOXA-22 and blaOXA-60 genes. As our WGS results show, the close aminoacid identities of OXA-444 protein to the OXA-60 and the presence of the serin-hydrolase class C beta-lactamase, may explain the carbapenem resistance (imipenem included) of the R. mannitolilytica isolate. These enzymes, in association with different mechanisms such as porin deficiency or overexpression of efflux pumps, may confer resistance to multiple classes of antibiotics.10,17
Aggregate data obtained from a literature review revealed a notable heterogeneity in the percentage of antibiotic resistance, as well as differences in the antibiotic susceptibility profiles of the two species. Although our analysis included only two isolates (very few compared to the literature data of 35 strains shown in Table 2) we did observe antibiotic resistance discrepancies regarding third generation cephalosporins and imipenem. Our patient was treated with ciprofloxacin and trimethoprim-sulfamethoxazole, both considered first-choice antibiotics. This turned out to be the best management of the patient; other treatment recommendations include third-generation cephalosporins or carbapenems, which would probably have not been able to eradicate the infection.11 Despite Ralstonia spp. are not recognized as major pathogens, their multidrug resistance, biofilm formation potential and ability to survive in the environment are factors that should never be underestimated. Clinicians and microbiologists should pay attention to the potential of these opportunistic bacteria able to cause severe bloodstream infections.
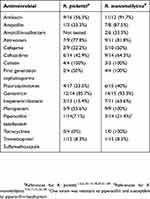 |
Table 2 Antimicrobial resistances identified in R. pickettii and R. mannitolilytica clinical isolates, obtained from a literature review |
Ethical statement
The patient provided written informed consent to describe the case details, and no institutional approval was needed for publication.
Acknowledgment
This work was supported by “Ricerca corrente” research funds from the Italian Ministry of Health.
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in regard to this work.
References
1. Bonatti H, Stelzmueller I, Laimer I, Obwegeser A. Ralstonia pickettii meningitis in a child with hydrocephalus. Eur J Pediatr Surg. 2009;19(5):341–342. doi:10.1055/s-0029-1202252
2. Choudhury H, Jindal A, Pathengay A, Flynn HW
3. Dotis J, Printza N, Orfanou A, Papathanasiou E, Papachristou F. Peritonitis due to Ralstonia mannitolilytica in a pediatric peritoneal dialysis patient. New Microbiol. 2012;35(4):503–506.
4. Vaneechoutte M, De Baere T, Wauters G, et al. One case each of recurrent meningitis and hemoperitoneum infection with Ralstonia mannitolilytica. J Clin Microbiol. 2001;39(12):4588–4590.
5. Gröbner S, Heeg P, Autenrieth IB, Schulte B. Monoclonal outbreak of catheter-related bacteraemia by Ralstonia mannitolilytica on two haemato-oncology wards. J Infect. 2007;55(6):539–544. doi:10.1016/j.jinf.2007.07.021
6. Mikulska M, Durando P, Pia Molinari M, et al. Outbreak of Ralstonia pickettii bacteraemia in patients with haematological malignancies and haematopoietic stem cell transplant recipients. J Hosp Infect. 2009;72(2):187–188. doi:10.1016/j.jhin.2009.02.016
7. Ryan MP, Adley CC. Ralstonia spp.: emerging global opportunistic pathogens. Eur J Clin Microbiol Infect Dis. 2014;33(3):291–304. doi:10.1007/s10096-013-1975-9
8. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. 2019. Available from: http://www.eucast.org/clinical_breakpoints/. Accessed February 13, 2019.
9. Rosa L, Cutone A, Coletti M, et al. Biotimer assay: a reliable and rapid method for the evaluation of central venous catheter microbial colonization. J Microbiol Methods. 2017;143:20–25. doi:10.1016/j.mimet.2017.10.002
10. Elsner HA, Dahmen GP, Laufs R, Mack D. Ralstonia pickettii involved in spinal osteitis in an immunocompetent adult. J Infect. 1998;36(3):352. doi:10.1016/S0163-4453(98)94999-4
11. Orme J, Rivera-Bonilla T, Loli A, Blattman NN. Native valve endocarditis due to Ralstonia pickettii: a case report and literature review. Case Rep Infect Dis. 2015;2015:324675.
12. Pan W, Zhao Z, Dong M. Lobar pneumonia caused by Ralstonia pickettii in a sixty-five-year-old Han Chinese man: a case report. J Med Case Rep. 2011;5:377. doi:10.1186/1752-1947-5-377
13. Segrelles-Calvo G, Sánchez Hernández A, Rey L. Bilateral pneumonia due to Ralstonia pickettii in immunocompetent patient. Med Clin (Barc). 2016;147(11):516–517. doi:10.1016/j.medcli.2016.06.037
14. Birlutiu RM, Roman MD, Cismasiu RS, et al. Sonication contribution to identifying prosthetic joint infection with Ralstonia pickettii: a case report and review of the literature. BMC Musculoskelet Disord. 2017;18(1):311. doi:10.1186/s12891-017-1624-z
15. Adley CC, Ryan MP, Pembroke JT, Saieb FM. Ralstonia pickettii: biofilm formation in high-purity water. In: McBain AJ, Allison DG, Pratten J, Spratt DA, Upton M, Verran J, editors. Biofilms: Persistence and Ubiquity. Cardiff: Biofilm Club; 2005:261–271.
16. Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78(3):510–543.
17. Lucarelli C, Di Domenico EG, Toma L, et al. Ralstonia mannitolilytica infections in an oncologic day ward: description of a cluster among high-risk patients. Antimicrob Resist Infect Control. 2017;6:20. doi:10.1186/s13756-017-0178-z
18. Tejera D, Limongi G, Bertullo M, Cancela M. Ralstonia pickettii bacteremia in hemodialysis patients: a report of two cases. Rev Bras Ter Intensiva. 2016;28(2):195–198. doi:10.5935/0103-507X.20160018
19. Chen YY, Huang WT, Chen CP, et al. An outbreak of Ralstonia pickettii bloodstream infection associated with an intrinsically contaminated normal saline solution. Infect Control Hosp Epidemiol. 2017;38(4):444–448. doi:10.1017/ice.2016.327
20. Maroye P, Doermann HP, Rogues AM, Gachie JP, Mégraud F. Investigation of an outbreak of Ralstonia pickettii in a paediatric hospital by RAPD. J Hosp Infect. 2000;44(4):267–272. doi:10.1053/jhin.1999.0691
21. Souza DC, Palmeiro JK, Maestri AC, et al. Ralstonia mannitolilytica bacteremia in a neonatal intensive care unit. Rev Soc Bras Med Trop. 2018;51(5):709–711. doi:10.1590/0037-8682-0118-2018
22. Ziganshina EE, Sharifullina DM, Lozhkin AP, Khayrullin RN, Ignatyev IM, Ziganshin AM. Bacterial communities associated with atherosclerotic plaques from russian individuals with atherosclerosis. PLoS One. 2016;11:e0164836. doi:10.1371/journal.pone.0164836
23. Spaziante M, Ceccarelli G, Al Moghazi S, Alessandri F, Venditti M. Specific dynamic of serum procalcitonin in critically ill patients affected by Gram-negative bacilli septic thrombophlebitis. Crit Care. 2018;22(1):178. doi:10.1186/s13054-017-1926-4
24. Ceccarelli G, Giuliano S, Falcone M, Venditti M. Follow-up blood cultures: a 2.0 diagnostic tool in patients with gram-negative bacteremia and septic thrombophlebitis. Clin Infect Dis. 2018;66(7):1154–1155.
25. Prior AR, Gunaratnam C, Humphreys H. Ralstonia species - do these bacteria matter in cystic fibrosis? Paediatr Respir Rev. 2017;23:78–83.
26. Lim CTS, Lee SE. A rare case of Ralstonia mannitolilytica infection in an end stage renal patient on maintenance dialysis during municipal water contamination. Pak J Med Sci. 2017;33(4):1047–1049. doi:10.12669/pjms.334.13112
27. Liu CX, Yan C, Zhang P, Li FQ, Yang JH, Li XY. Ralstonia mannitolilytica-induced septicemia and homology analysis in infected patients: 3 case reports. Jundishapur J Microbiol. 2016;9(7):e34373. doi:10.5812/jjm.34373
28. Mukhopadhyay C, Bhargava A, Ayyagari A. Ralstonia mannitolilytica infection in renal transplant recipient: first report. Indian J Med Microbiol. 2003;21(4):284–286.
29. Zong ZY, Peng CH. Ralstonia mannitolilytica and COPD: a case report. Eur Respir J. 2011;38(6):1482–1483. doi:10.1183/09031936.00046011
30. Shankar M, Rampure S, Siddini V, Ballal HS. Outbreak of Ralstonia mannitolilytica in hemodialysis unit: a case series. Indian J Nephrol. 2018;28(4):323–326.
31. Barbut F, Kosmann MJ, Lalande V, Neyme D, Coppo P, Gorin NC. Outbreak of Ralstonia pickettii pseudobacteremia among patients with hematological malignancies. Infect Control Hosp Epidemiol. 2006;27(6):642–644. doi:10.1086/505100
32. Forgie S, Kirkland T, Rennie R, Chui L, Taylor G. Ralstonia pickettii bacteremia associated with pediatric extracorporeal membrane oxygenation therapy in a Canadian hospital. Infect Control Hosp Epidemiol. 2007;28(8):1016–1018. doi:10.1086/518754
33. Kendirli T, Ciftçi E, Ince E, et al. Ralstonia pickettii outbreak associated with contaminated distilled water used for respiratory care in a paediatric intensive care unit. J Hosp Infect. 2004;56(1):77–78. doi:10.1016/j.jhin.2003.09.011
34. Kimura AC, Calvet H, Higa JI, et al. Outbreak of Ralstonia pickettii bacteremia in a neonatal intensive care unit. Pediatr Infect Dis J. 2005;24(12):1099–1103. doi:10.1097/01.inf.0000190059.54356.f3
35. Kismet E, Atay AA, Demirkaya E, et al. Two cases of Ralstonia pickettii bacteremias in a pediatric oncology unit requiring removal of the Port-A-Caths. J Pediatr Hematol Oncol. 2005;27(1):37–38. doi:10.1097/01.mph.0000149960.89192.b0
36. Sharma D, Sharma P, Soni P, Gupta B. Ralstonia picketti neonatal sepsis: a case report. BMC Res Notes. 2017;10(1):28. doi:10.1186/s13104-016-2347-1
37. Strateva T, Kostyanev T, Setchanova L. Ralstonia pickettii sepsis in a hemodialysis patient from Bulgaria. Braz J Infect Dis. 2012;16(4):400–401. doi:10.1016/j.bjid.2012.06.010
38. Zellweger C, Bodmer T, Täuber MG, Mühlemann K. Failure of ceftriaxone in an intravenous drug user with invasive infection due to Ralstonia pickettii. Infection. 2004;32(4):246–248. doi:10.1007/s15010-004-3033-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
