Back to Journals » International Medical Case Reports Journal » Volume 15
A Case of Intraocular Lymphoma Diagnosed by Subretinal Fluid Biopsy
Authors Inami W , Shibuya M, Kumagai T, Makita J, Shinoda K
Received 31 October 2021
Accepted for publication 9 March 2022
Published 21 March 2022 Volume 2022:15 Pages 111—115
DOI https://doi.org/10.2147/IMCRJ.S345149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Wataru Inami, Masayuki Shibuya, Tomoyuki Kumagai, Jun Makita, Kei Shinoda
Department of Ophthalmology, Saitama Medical University Faculty of Medicine, Saitama, Japan
Correspondence: Kei Shinoda, Department of Ophthalmology, Saitama Medical University Faculty of Medicine, Saitama, Japan, Tel +81-49-276-1250, Email [email protected]
Abstract: Although intraocular lymphoma (IOL) mainly has have vitreous opacity and subretinal infiltration, its clinical symptoms are diverse. We report a case of IOL that mainly showed exudative retinal detachment in which analysis of IgH gene rearrangement (AIGHR) of the collected subretinal fluid sample was useful for diagnosis. A 77-year-old woman developed decreased left visual acuity for 1 month. She had been treated for dermatomyositis, diabetes mellitus, and right parotid tumor for 3 years. Visual acuity was 0.1 OD and counting fingers OS. Slit-lamp examination showed grade 4 (Emery-Little classification) nuclear cataract in both eyes and keratoprecipitates and tan vitreous opacity in the left eye. Fundoscopy details were unclear except for a vaguely observable optic nerve head due to yellow-brown vitreous opacity, which we judged as an old vitreous hemorrhage. Phacovitrectomy was performed and almost total retinal detachment was found, except for a part of the superior periphery. Since no retinal break was found and a wide range of thin membrane-like tissue was found on the surface of the retina, the surgeon suspected primary IOL and performed unplanned biopsy. The peripheral vitreous was collected as a sample, and then the subretinal fluid was collected through an intentional break to prevent mixing with other fluids. The subretinal strand was gently removed and collected. Cytology showed class III, the IL10/IL6 ratio was low, and AIGHR was positive. Postoperatively, fundus autofluorescence showed no abnormality, no leakage was observed on fluorescein and indocyanine green angiography, and the location of typical infiltration lesions under the retina was unclear. There were no positive findings on systemic examinations and a diagnosis of primary IOL was made. The main symptoms of this case were vitreous opacity and exudative retinal detachment, and AIGHR using subretinal fluid was useful for diagnosis.
Keywords: primary intraocular lymphoma, exudative retinal detachment, IgH gene rearrangement, pars plana vitrectomy
Introduction
Because the main presentations of primary intraocular lymphoma (PIOL) are often vitreous opacity and the formation of subretinal infiltrative lesions, its diagnosis has conventionally been based on demonstrating cell morphology obtained by pars plana vitrectomy (PPV).1 However, the paucicellular specimens with fragile populations of lymphocytes retrieved through PPV make morphological diagnosis difficult. Tests using biopsy specimens include cytology, ratio of interleukin (IL)-10 to IL-6, flow cytometry, and immunoglobulin heavy chain (IgH) gene rearrangement. Clinical findings are normally combined to make a comprehensive diagnosis.1–3 We report a case that was initially considered to be an old vitreous hemorrhage and performed PPV, but suspected PIOL during the operation and performed a biopsy. Analysis of the IgH gene rearrangement (AIGHR) of the collected subretinal fluid samples was useful for diagnosis.
Case Report
A 77-year-old woman visited her local doctor complaining of decreased visual acuity in her left eye for 1 month. She was referred to our Department for further examination and treatment of vitreous opacity in the left eye. She has been on treatment for dermatomyositis, diabetes mellitus, and right parotid tumor with prednisolone (6 mg/day) and tacrolimus hydrate (2 mg/day) for 3 years.
On the initial visit, the best-corrected visual acuity was 0.04, counting fingers, and the intraocular pressure was 17.0 mmHg and 13.3 mmHg in the right and left eyes, respectively. Slit-lamp microscopy revealed Grade 4 (Emery-Little classification) nuclear cataracts in both eyes, and kerato-precipitates and tan vitreous opacity in the left eye. The left optic nerve head was vaguely observed due to vitreous opacity. B-mode echography of the left eye showed relatively dense vitreous opacity and membranous material on the surface of the retina.
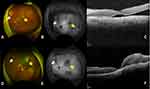
We considered the tan-like vitreous opacity to be an old vitreous hemorrhage and performed a phacovitrectomy. When the cataract and vitreous opacity were removed, almost total retinal detachment, except for a part of the superior periphery, was observed (Figure 1A). Since no retinal break was found and a wide range of thin membrane-like tissue was found on the surface of the retina, the surgeon considered possible PIOL for the first time and performed an unplanned biopsy. The peripheral vitreous was collected as a sample that had already been diluted with irrigating fluid. Therefore, the subretinal fluid was collected through an intentional break to prevent mixing with other fluids (Figure 1B). Silicone oil was injected at the end of the surgery. Because the subretinal fluid had slightly increased and involved the macula (Figure 2A and C), re-PPV was performed to further aspirate the subretinal fluid, and the subretinal strand close to the inferior arcade vessel was gently pulled out and collected as a sample. These samples were submitted for cytology, IL-10/IL-6 ratio measurement, and AIGHR. Cytology revealed class II vitreous specimens and class III subretinal fluid (mainly lymphocytes with mild karyotype but no atypia). The pathologist ascertained that the specimen from subretinal fluid was class III, considering the possibility of low-grade MALT lymphoma, which suggests severe dysplasia, carcinoma in situ, or cancer. Cytokine measurements showed that the vitreous fluid was unmeasurable, and the subretinal fluid had a low IL10/IL6 ratio <1.0. The results of AIGHR in the subretinal fluid were positive for monoclonality. The subretinal fluid was gradually reduced, and subtenon injection of triamcinolone acetonide was effective for macular edema. Although the outer retinal layer was atrophic in the macular area, visual acuity in the left eye improved to 0.3 (Figure 2F). Fundus autofluorescence imaging showed localized hypofluorescent areas corresponding to intentional breaks and surrounding photocoagulated scars (Figure 2D and E). No leakage was observed on fluorescein and indocyanine green fluorescence angiography, and the location of typical subretinal infiltration was unclear. The right eye underwent cataract surgery and visual acuity improved to 0.9. Fundus examination revealed no abnormal findings including IOL-related changes. There were no positive findings on head-enhanced magnetic resonance imaging or whole-body fluorodeoxyglucose positron emission tomography-computed tomography, and a diagnosis of PIOL was made. No relapse of intraocular inflammation was observed, and the patient was carefully monitored.
Discussion and Conclusion
In the diagnosis of malignant IOL, the sensitivities of individual detection methods, such as cytology, AIGHR, IL-10/IL-6 ratio, and clinical findings, are not high. The specificities of vitreous fluid cytopathology, flow cytometry, and AIGHR are all 1.0, with sensitivity values of 0.24, 0.36, and 0.64, respectively.3 Other authors have assessed cytology, AIGHR, IL-10/IL-6 ratio, and clonal B-cells on flow cytometry and concluded that malignant cytology or positive results for two or more of four tests may be adequate for VRL diagnosis.4 In our case, AIGHR and cytology were positive and met this criterion. It may be possible to supplement the detection sensitivity by collecting multiple biopsy materials, such as vitreous humor and subretinal fluid, and performing multiple tests.
The main symptoms of this case were vitreous opacity and exudative retinal detachment, and AIGHR using subretinal fluid as a sample was useful for identifying the cause. When looking at various tests, the results of the subretinal fluid sample were more sensitive than those of the vitreous sample in both cytology and cytokine measurement. Ciulla et al reported a case that was negative for two vitreous biopsies and was diagnosed with PIOL by cytology from a subretinal fluid sample, and a concurrent retinal biopsy failed to confirm the diagnosis.5 In the present case, since IOL was suspected based on the intraoperative findings, the collected vitreous specimens were diluted, which may have affected the test sensitivity, but the information from the subretinal fluid was extremely useful for diagnosis.
A subretinal mass or infiltrate is one of the characteristics of IOL.5 And biopsy of subretinal fluid or tissue using fine needles has been reported to be useful.6,7 In the current case, careful attention was paid to avoid contamination, subretinal fluid was aspirated, and cytology and AIGHR were performed, which helped in the diagnosis. It is not possible to make a definitive diagnosis using a single method. In the current case, we used a 25-gauge tool for the subretinal fluid biopsy. However, the 40-gauge needle is better from the viewpoint of self-closing of the intentional break. The diagnostic techniques applied depend on factors such as access to the technique and the operator’s knowledge, clinical situation, and available resources. Diagnosis relies on the integration of all information available to pathologists and clinicians, including ancillary research techniques and observation of disease progression. And retinal detachment was suspected in the patients with IOL. If subretinal infiltrate or exudative detachment with vitreous opacity is observed, it may be useful to perform cytodiagnosis and AIGHR using subretinal fluid as a sample, even with only a small amount.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
The ethics committee of Saitama Medical University ruled that approval was not required for this study. Written informed consent was obtained from the patient in this case report and for the publication of this article and accompanying images. A copy of the written consent is available for review by the editor of this journal.
Acknowledgment
The authors thank Editage for English language review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported in part by the Japan Society for the Promotion of Science (JSPS; KAKENHI grant number: 21K16904).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Mastropasqua R, Thaung C, Pavesio C, et al. The role of chorioretinal biopsy in the diagnosis of intraocular lymphoma. Am J Ophthalmol. 2015;160(1127–1132.e1). doi:10.1016/j.ajo.2015.08.033
2. Wang Y, Shen D, Wang VM, Sen HN, Chan CC. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int J Mol Sci. 2011;12:5684–5697. doi:10.3390/ijms12095684
3. Baehring JM, Androudi S, Longtine JJ, et al. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer. 2005;104:591–597. doi:10.1002/cncr.21191
4. Tanaka R, Kaburaki T, Taoka K, et al. More accurate diagnosis of vitreoretinal lymphoma using a combination of diagnostic test results: a prospective observational study. Ocul Immunol Inflam. 2021;1:1–7. doi:10.1080/09273948.2021.1873394
5. Ciulla TA, Pesavento RD, Yoo S. Subretinal aspiration biopsy of ocular lymphoma. Am J Ophthalmol. 1997;123:420–422. doi:10.1016/s0002-9394(14)70150-3
6. Levy-Clarke GA, Byrnes GA, Buggage RR, et al. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a subretinal lesion. Retina. 2001;21:281–284. doi:10.1097/00006982-200106000-00023
7. Rao M. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a subretinal lesion. Retina. 2002;22:512–513. doi:10.1097/00006982-200208000-00025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.