Back to Journals » OncoTargets and Therapy » Volume 11
A case of interstitial lung disease with alveolar hemorrhage induced by pembrolizumab
Authors Sugano T, Seike M, Noro R, Kaburaki S, Tozuka T , Takahashi A, Takano N , Tanaka T, Kashiwada T, Takeuchi S, Minegishi Y, Saito Y, Kubota K, Terasaki Y , Gemma A
Received 27 March 2018
Accepted for publication 31 May 2018
Published 17 September 2018 Volume 2018:11 Pages 5879—5883
DOI https://doi.org/10.2147/OTT.S169321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Teppei Sugano,1 Masahiro Seike,1 Rintaro Noro,1 Syota Kaburaki,1 Takehiro Tozuka,1 Akihiko Takahashi,1 Natsuki Takano,1 Toru Tanaka,1 Takeru Kashiwada,1 Susumu Takeuchi,1 Yuji Minegishi,1 Yoshinobu Saito,1 Kaoru Kubota,1 Yasuhiro Terasaki,2 Akihiko Gemma1
1Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan; 2Department of Analytic Human Pathology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan
Abstract: We herein describe the case of a 67-year-old woman with advanced lung adenocarcinoma who developed interstitial lung disease (ILD) with alveolar hemorrhage induced by pembrolizumab. She received four courses of pembrolizumab therapy and achieved a partial response. She had no respiratory symptoms; however, chest radiography and computed tomography (CT) revealed ground-glass opacities (GGOs) and crazy-paving pattern. Based on findings of bloody bronchoalveolar lavage fluid and transbronchial lung biopsy samples, pembrolizumab-induced ILD with alveolar hemorrhage was diagnosed. Corticosteroid therapy rapidly improved alveolar hemorrhage and regressed GGOs on CT scan. This is the first report on ILD with alveolar hemorrhage induced by pembrolizumab.
Keywords: pembrolizumab, PD-1, pneumonitis, alveolar hemorrhage
Introduction
Immune checkpoint inhibitors (ICIs) targeting PD-1, such as nivolumab and pembrolizumab, have emerged as new therapeutic agents for advanced nonsmall-cell lung cancer (NSCLC). Although these ICIs have demonstrated remarkable antitumor-specific immune responses, they can also cause immune-related adverse events (irAEs) involving the skin, liver, gastrointestinal and endocrine systems, and lung.1–3 Among irAEs, interstitial lung disease (ILD) is known to lead to serious lung injury or life-threatening complications. Here, we report the first case of pembrolizumab-induced ILD with alveolar hemorrhage in a lung adenocarcinoma patient.
Case report
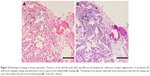
A 67-year-old female former smoker was diagnosed with adenocarcinoma on the basis of pathological examination of transbronchial biopsy specimens. Distant metastases were not detected by the whole-body assessment. She underwent a right upper lobectomy with systematic lymph node dissection for lung adenocarcinoma (pT2aN0M0 stage IB: 7th edition of UICC TNM staging). Two years after the surgery, she developed numbness in both feet and was admitted to the emergency room. Spine MRI and chest computed tomography (CT) showed obvious nerve compression due to intradural extramedullary spinal metastasis, suggesting recurrence of her lung cancer. She underwent emergency palliative laminectomy decompression surgery. The pathological features and molecular profiles of the resected intradural extramedullary spinal tumor were reviewed. We diagnosed metastasis from lung adenocarcinoma; neither a mutated EGFR gene nor an ALK fusion gene was detected. Immunohistochemistry revealed high expression of PD-L1 showing a tumor proportion score of 50%–74% on the tumor cell membrane. One month after the palliative surgery, the patient’s performance status improved from 3 to 1. However, as left pleural effusion, left hilar lymphadenopathy, and left lower tumor progressed (Figure 1), pembrolizumab treatment (200 mg/day) was initiated. After four cycles of pembrolizumab treatment, she did not have any respiratory symptoms, including coughing, hemoptysis, and dyspnea. However, chest X-ray revealed ground-glass opacities (GGOs) and consolidation in both the lungs. Chest CT showed GGOs and consolidation in both the lungs around the bronchial vascular bundles, along with a crazy-paving pattern (Figure 2A–C). A hematological examination showed increased inflammatory response (white blood cells 8,400/μL and serum C-reactive protein 4.7 mg/dL) and anemia (serum hemoglobin of 9.7 g/dL). Although KL-6 was not high (380 U/mL), SP-D was increased (351 ng/mL). The patient’s serum antibody titers against Mycoplasma pneumoniae and Chlamydia pneumoniae were not elevated. The patient’s urine was negative for pneumococcal and Legionella pneumophila serotype 1 antigens. Serologic data revealed no abnormal findings suggestive of collagen vascular diseases, including anti-neutrophil cytoplasmic antibody (ANCA), and no microorganisms grew on a sputum culture. Fiberoptic bronchoscopy was performed. Bronchoalveolar lavage (BAL) fluid from the right lower lobe gradually became bloody (Figure 3) with 45% lymphocytes (24.0% CD4, 71.4% CD8), 40% macrophages, 15% neutrophils, and 0% eosinophil. No microorganisms grew on a BAL fluid culture, and no evidence of malignancy was apparent. A transbronchial lung biopsy (TBLB) specimen obtained from the right lower lobe showed thickening of the alveolar walls with myxofibrous and lymphocytic infiltration changes (Figure 4). Although we could not find hemosiderin-laden macrophages in the TBLB specimens, agglutination of red blood cells with focal coagulate change was observed in the air spaces of the alveoli. We diagnosed pembrolizumab-induced ILD with alveolar hemorrhage. After fiberoptic bronchoscopy, oxygen desaturation was found, and oral prednisolone was started at 1.0 mg/kg/day for 2 weeks (initial dose: 40 mg/day) and then the dose was gradually decreased by 5 mg/day every week until reaching a dose of 20 mg/day. The patient’s radiographic abnormal findings resolved rapidly (Figure 5), and oral prednisolone was tapered and then discontinued 3 months later without a recurrence of pneumonitis. The patient showed partial responses to pembrolizumab treatment; however, we did not try a challenge readministration of pembrolizumab.
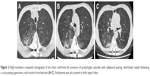
  | Figure 1 Computed tomography of the chest before treatment of pembrolizumab. Left pleural effusion, left hilar lymphadenopathy, and left lower tumor were observed (A and B). |
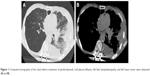
  | Figure 3 The bronchoalveolar lavage fluid gradually became bloody from the left tube to the right tube. |
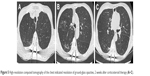  | Figure 5 High-resolution computed tomography of the chest indicated resolution of ground-glass opacities, 2 weeks after corticosteroid therapy (A–C). |
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
In the case described herein, ILD with alveolar hemorrhage appeared after four cycles of pembrolizumab treatment. The diagnosis of alveolar hemorrhage induced by pembrolizumab was definitive based on the findings of BAL and pathological features without respiratory tract infection, collagen vascular diseases, or congestive heart failure.
Drug-induced alveolar hemorrhage is a serious and life-threatening event resulting in disruption of the alveolar–capillary basement membrane. This disruption is caused by either direct pharmacological effects on alveolar micro-capillaries or indirect effects via the stimulation of inflammatory reactions. Direct effects include a vasculitis-like process that is ANCA-positive or acute lung injury. ANCA-related alveolar hemorrhage is known to be induced by propylthiouracil, penicillamine, and tetracyclines; acute lung injury has been linked to antitumor drugs such as irinotecan, gemcitabine, and gefitinib.4 In this case, histological examination showed active fibrosing alveolitis; therefore, we concluded that alveolar hemorrhage might have developed through acute lung injury by pembrolizumab. The pathogenesis of this case was unknown, because the patient was negative for common autoimmune antibodies including ANCA. Therefore, further research will be necessary to find unknown antibodies and examine the mechanism of ICI-induced lung injury related to alveolar hemorrhage.
Our case developed ILD at 3.5 months after introducing pembrolizumab. Delaunay et al showed the median time to onset of ICI-induced ILD was 2.3 months (<2 months in 42.2%, 2–4 months in 26.6%, and 4–6 months in 17.2%).5 The incidence of any-grade pembrolizumab-induced ILD was 5.8%, and that of grade 3 or 4 ILD was 2.6% in the Phase III KEYNOTE-024 trial.2 In contrast, in the Phase II/III KEYNOTE-010 trial, any-grade pneumonitis occurred in 4%–5% of patients, and six out of 691 patients died from pulmonary toxicity.6 In the Phase I KEYNOTE-001 trial, pneumonitis occurred in 18 (3.6%) patients, with one death attributed to pneumonitis.3,7 In these clinical trials of pembrolizumab, the majority of pneumonitis cases were reported as mild to moderate, but they had the potential to lead to life-threatening complications. Therefore, careful monitoring of the patients for any respiratory symptoms is important during pembrolizumab treatment, especially within 6 months.
ILD induced by anti-PD-1 inhibitors is a well-known complication; however, the detailed clinical, radiographic, and pathological manifestations are unclear. Nishino et al reported on two cases of nivolumab-induced ILD in advanced NSCLC patients demonstrating a radiographic pattern of cryptogenic organizing pneumonia (COP).8 Naidoo et al showed that the radiologic features of ILD causing PD-1 or PD-L1 inhibition were COP-like (five of 27 [19%]), with GGOs (10 of 27 [37%]), interstitial involvement (two of 27 [7%]), and hypersensitivity (six of 27 [22%]). The histopathological findings were cellular interstitial pneumonitis (four of 11) and organizing pneumonia (OP) (three of 11).9 We previously reported on the occurrence of ILD with OP findings after nivolumab therapy.10 However, pembrolizumab-induced ILD has not been well characterized.
Treatment strategies for immunotherapy-induced ILD were described by Puzanov et al.11 Drug withdrawal is required for most of the patients with all grades of pneumonitis,11 and patients with grade 2 or higher are prescribed oral/intravenous corticosteroids. When our case was diagnosed with pneumonitis, she did not have any respiratory symptoms. On the next day of conducting fiberoptic bronchoscopy, pulse oximetry saturation (SpO2) decreased by around 90%. Therefore, we initiated corticosteroid therapy. For patients with grade 1–2 pneumonitis, drug rechallenge is considered if symptoms and imaging abnormalities are resolved. Delaunay et al reported three of 10 patients who restarted immunotherapy developed recurrent ILD.5 Because the risk of second ILD is high and shrinking of tumor size has continued, treatment of pembrolizumab has not been restarted.
Identifying the risk factors for the induction of ILD by PD-1 inhibitors is important. In an interim analysis based on a data set of 1,005 Japanese patients,12 the risk factors for the occurrence of ILD were: age (≥75 years), abnormal chest CT findings other than lung cancer, and treatment line (second line). Pembrolizumab-induced ILD occurred more frequently in patients with a history of asthma/COPD than in those without that history (5.4% vs 3.1%) and more frequently in patients with a history of prior thoracic radiation than in patients without radiation (6.0% vs 2.6%).7 In this case, the patient also had a history of COPD; therefore, we suggest careful management of patients with COPD while they undergo treatment with PD-1 inhibitors.
In conclusion, we have reported the first case of ILD with alveolar hemorrhage induced by pembrolizumab. Although ICIs are very promising antitumor treatments, the possibility of drug-induced ILD should be considered even if the patients have no specific respiratory clinical symptoms.
Disclosure
Masahiro Seike, Kaoru Kubota, and Akihiko Gemma have received honorarium from Merck Sharp & Dohme. The authors report no other conflicts of interest in this work.
References
Borghaei H, Paz-Ares L, Horn LN, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. | ||
Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. | ||
Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. | ||
Kim SH, Minami S, Ogata Y, Yamamoto S, Komuta K. Diffuse alveolar hemorrhage induced by irinotecan for a patient with metastatic thymic carcinoma: a case report and literature review. Intern Med. 2017;56(13):1697–1700. | ||
Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(2):1700050. | ||
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. | ||
Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016;21(5):643–650. | ||
Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res. 2016;4(4):289–293. | ||
Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2016;35(7):709–717. | ||
Kashiwada T, Saito Y, Minegishi Y, et al. Granuloma-forming interstitial pneumonia induced by nivolumab: a possible immune-related adverse event of the lung. Int Cancer Conf J. 2017;6(3):131–134. | ||
Puzanov I, Diab A, Abdallah K, et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. | ||
Kenmotsu H, Sakai F, Kato T, Kusumoto M, Baba T, Kuwano K. Nivolumab-induced interstitial lung disease in Japanese patients with non-small cell lung cancer: a study on risk factors using interim results of post-marketing all-case surveillance. J Clin Oncol. 2017;35(15 Suppl):9078. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.